Application Note: 111 – Measuring Surface Charge Density: A New Application with SPR
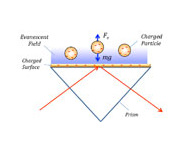
Surface charge density is a basic quantity that is directly relevant to many phenomena, from surface interactions to DNA hybridization. Measurement and quantification of surface charge density can lead to a better understanding of biomolecular interactions on surfaces. To date, different techniques have been developed to measure surface charge density, including potentiometric titration, atomic force microscopy and reflection interference
Application Note: 110 – Studying Protein Adsorption Properties with SPR
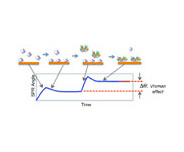
Surface Plasmon Resonance (SPR) can be applied as a convenient, sensitive and label-free technique to study various surface phenomena. One such example is the Vroman effect exhibited by protein adsorption onto surfaces. This important effect arises from the fact that protein adsorption capability onto a surface depends on motility, which is intimately related to its molecular weight. In general, a high molecular weight (HMW) protein adsorbs
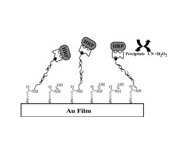
Application Note: 109 – Application of SPR to Bacteriology: Endotoxin/Protein Interaction Studies
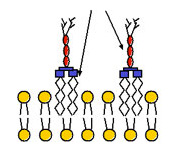
Endotoxin (commonly referred to as lipopolysaccharide in bacteriology) is associated with the outer membrane of Gram-negative bacterial pathogens such as Escherichia coli, Salmonella, Shigella, and Pseudomonas [1-2]. The interaction between endotoxin and bacterial cell surface is schematically depicted in Figure 1. Endotoxin elicits a series of pleiotropic effects on cells or organisms and is therefore harmful to most mammals
Application Note: 108 – SPR as a Chromatographic Detector: Separation and Label-Free Detection of Biomolecules
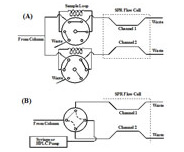
In recent years, SPR has been used as an alternative detector for monitoring elution of a variety of species (e.g., polysaccharides and proteins [1-4]) out of liquid chromatographic (LC) columns. In addition to simplicity and fast speed the SPR detector can detect any eluent, as long as its index of refraction is different from that of the carrier buffer. The open design of the BI-SPR instruments allows users to conveniently connect
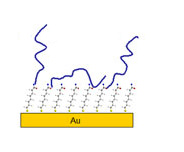
Application Note: 107 – Binding Kinetics Analysis with SPR: Interaction between Bovine Serum Albumin (BSA) and Anti-BSA
In restriction digestion, BSA has been used to stabilize enzymes during DNA digestion. It is also widely used as a biomolecule to block active sites on surfaces. Formation of the anti-BSA/BSA immune complex is relevant to studies of the receptor site of the red blood cells..
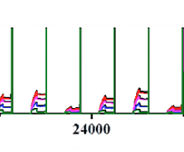
Application Note 106: Flow Injection-SPR: Sensitive and Sequence-Specific DNA Assays
SPR (SPR) has been demonstrated as a powerful technique for rapid, sensitive, and label-free genetic analysis [1-5]. When the sensor surface is coated with a single sensing (probe) DNA, SPR can be used for both affinity binding studies (i.e., kinetic measurements) and concentration detection of a target DNA. However, the concentration detection levels for SPR and SPR imaging are typically at low nanomolar (nM) [3, 4],
Application Note: 105 – Protein/Drug Interaction: Ferulic Acid and Bovine Serum Albumin
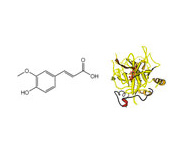
SPR has been demonstrated to be a powerful optical technique for bioaffinity studies at the solid/solution interface. In general, SPR measures the change in refractive index originated from the binding of a solution species with a molecule pre-immobilized onto the SPR sensor chip. The advantages of SPR include its simplicity, high sensitivity, obviation of sample labeling, and amenability for real-time analysis
Application Note 104: Flow-Injection SPR: Sensitive Determinations of Heavy Metal Ions
Flow-injection SPR has been demonstrated as a viable alternative for sensitive detection of heavy metal ions at trace levels [1-4]. The impetus behind using SPR for elemental analysis stems from its high sensitivity, simplicity, compact design (for possible field-based work), and universal detection mechanism (e.g., any elemental species adsorbed onto the SPR sensor can cause a detectable signal). The challenge, however, is to prepare a chemically or
Application Note 103: Electrochemical SPR: Metal Deposition/Stripping and Reorganization of Electroactive Organic Thin Films
In addition to studies of redox-induced conformational changes of surface-bound proteins (cf. Application Note #102), electrochemical SPR can be used to quantify the amount of metal electrodeposited onto a surface and reorganization of organic thin films upon redox reactions. A BI-SPR 1000 was used to quantify the amount of Cu deposited and stripped during Cu2+ reduction/Cu oxidation in a CuSO4 solution
Application Note 102: Electrochemical SPR: Redox-Induced Protein Conformational Changes
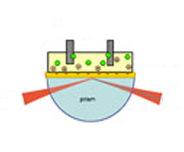
Both electrochemical and surface plasmon resonance (SPR) techniques measure various processes taking place at or near an electrode surface. Combining the two techniques allows one to obtain new insight into these interfacial processes. One example is redox-induced conformational changes in surface-bound protein molecules. However, most SPR setups are not suitable for monitoring fast kinetics or small thickness variations
