In addition to studies of redox-induced conformational changes of surface-bound proteins (cf. Application Note #102), electrochemical SPR can be used to quantify the amount of metal electrodeposited onto a surface and reorganization of organic thin films upon redox reactions. A BI-SPR 1000 was used to quantify the amount of Cu deposited and stripped during Cu2+ reduction/Cu oxidation in a CuSO4 solution (FIG. 1). In performing this experiment, a polyetheretherketone cell with an internal volume of 0.5 mL was used. A Pt wire and an Ag/AgCl, which fit tightly into the holes in the cell, served as the auxiliary and reference electrodes, respectively [1].
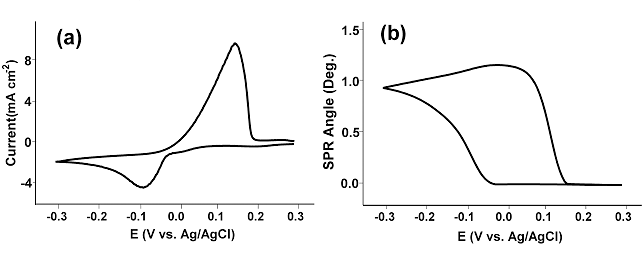
FIG. 1 (a) Cyclic voltammogram (a) and SPR angle shift (b) simultaneously recorded from a 5 mM CuSO4/0.1 M H2SO4solution at 50 mV/s at gold sensor chip.
Integrating charges under the reduction peak in FIG. 1a yielded the thickness of the deposited copper film to be approximately 13 nm. On the basis of the Fresnel equation and a four-layer (glass/Au/Cu/H2O) model for the Au sensor chip (FIG. 1b), the thicknesses of the copper film was found to be 15 nm, which agrees with the voltammetric data remarkably well.
In a separate experiment, electrochemical SPR was used to investigate the changes in orientations of ferrocene (Fc)-terminated alkanethiol self-assembled monolayers (SAMs)[2]. Such an electroactive organic thin film is a model system for probing interfacial electron transfer reactions [3], studies of molecular electronics, and construction of liquid crystal devices [4]. The potential at which Fc groups are oxidized is more positive than the point of zero charge. As a result, the ferrocenium cations are repelled by the positively charged electrodes. As shown by the schematics (FIG. 2), such repulsion changes the orientation or reorganization of the Fc-terminated alkanethiol SAM, decreasing the tilt angle of the alkyl chain. To compensate the charges at the SAM terminus, anions in the solution will form ion-pairs with the ferrocenium cations. This ion-pairing process results in a change in the refractive index of the SAM. The film reorganization and ion-pairing processes can be monitored by electrochemical SPR in real time (FIG 3). By quantifying the number of ions and the extent of solvation involved in the ion-pairing process, the infinitesimal changes shown in the two different orientations can be accurately determined.
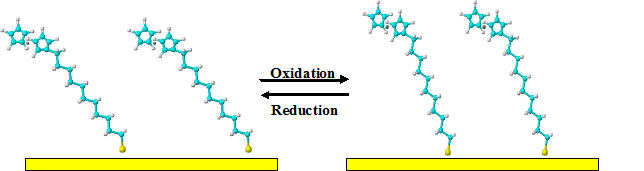
FIG. 2 A schematic representation of the reorganization of the 11-ferrocenylundecanethiol (FcC11SH) self-assembled monolayer upon ferrocene oxidation and the subsequent ion-pairing process. The positive charges on the electrode surface and conversion of ferrocene to ferrocenium cations lead to a decrease in the tilt angle of the alkanethiol chain.
The film thickness variation of the FcC11SH SAM in 0.1 M HClO4 was deduced to be 0.09 nm. This is in good agreement with the ellipsometric results [5]. The ability of determining such tiny thickness variations demonstrates the superb sensitivity of SPR for interrogating orientation changes associated with ultrathin organic films and the amenability of SPR for in-situ coupling with electrochemistry.
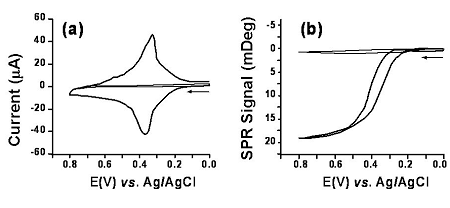
FIG. 3 Cyclic voltammogram (a) of a FcC11SH SAM in 0.1 M HClO4 and the corresponding SPR dip shift-potential diagram (b). The voltammogram and the SPR dip shift-potential diagram of a hexanethiol SAM (thinner curves in both panels) were also shown. Arrows indicate the scan direction and the scan rate was 50 mV/s.3
Author: Nguyen Ly | Biosensing Instrument | Published May 1, 2010
DOWNLOAD PDF
Download a PDF of Application Note 103: Electrochemical SPR: Metal Deposition/Stripping and Reorganization of Electroactive Organic Thin Films
- P. Zhai, J. Guo, J. Xiang, and F. Zhou, J. Phys. Chem. C 2007, 111, 981.
- X. Yao, J. Wang, F. Zhou, J. Wang, N. Tao, J. Phys. Chem. B 2004, 108:7206-7212.
- D. A. Brevnov, H. O. Finklea, H. V. Ryswyk, J. Electroanal.Chem. 2001, 500, 100-107.
- Y. Luk, N. L. Abbott Science 2003, 301, 623-626.
- T. Ohtsuka, Y. Sato, K. Uosaki, Langmuir 1994, 10, 3658-3662.
