Flow-injection SPR has been demonstrated as a viable alternative for sensitive detection of heavy metal ions at trace levels [1-4]. The impetus behind using SPR for elemental analysis stems from its high sensitivity, simplicity, compact design (for possible field-based work), and universal detection mechanism (e.g., any elemental species adsorbed onto the SPR sensor can cause a detectable signal). The challenge, however, is to prepare a chemically or biologically modified sensor chip that is specific to a given analyte without interferences from other species present in the sample solution. Zare and coworkers have showed that an alkanedithiol-covered gold surface is selective to mercury ion (Hg2+) [1], and Forzani et al. attached oligopeptides onto gold films for selective determinations of Ni2+ and Cu2+ [2]. Zhang et al. recently constructed a sensor surface covered with apo-metallothionein (i.e., metal-free metallothionein, a cysteine-rich protein) for selective determinations of Hg2+ and Cd2+ [3]. The principle behind this detection mechanism is illustrated in FIG. 1. As metal ions are complexed by the apo-metallothionein (apo-MT) molecules, the resultant protein conformational changes can be sensitively measured by SPR. The extent of the conformational changes (or thickness variation of the immobilized proteins) is proportional to the amount of metal ions in the sample solution. This method yielded detection levels for Cd2+ and Hg2+ at 0.1 µM and 5 µM, respectively [3].
The two sensorgrams depicted in FIG. 2 suggest that the apo-MT molecules have a stronger binding affinity with Hg2+ than with Cd2+ . When the Cd2+ injection was followed by the introduction of Hg2+ into the flow cell, the Hg2+ signal was much greater than the Cd2+ signal, even though both metal ions were of the same concentration. If the injection order was reversed (inset of FIG 2), the injection of Cd2+ into the cell housing a MT sensor chip that had already been exposed to Hg2+ did not yield appreciable SPR dip shifts.
A BI-SPR 1000 system, equipped with a dual-channel flow cell and a PDMS (polydimethylsiloxane) gasket, was also used for the detection of Hg2+ at a hexanedithiol-modified gold sensor chip. A 10-mM acetate buffer (pH 4.5) was used as the running buffer and a flow rate of 50 μL/min was used. Mercury standards of various concentrations were analyzed by injecting 20 μL of sample into the flow cell. FIG. 3a overlaid two representative sensorgrams that show the complexation of Hg2+ at two different concentrations by the surface-bound hexanedithiol. The calibration curve (FIG. 3b) exhibited excellent linearity between sub-μM to mM (R2 = 0.999). As demonstrated by Zare and coworkers, a carefully designed protocol based on this approach can lead to a detection level for Hg2+ down to sub-nM (1).
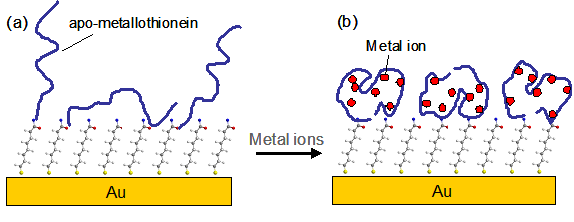
FIG. 1 Schematic representation of the conformation change of apo-MT upon metal binding. The black dots in (b) represent the metal ions. Notice that the predominant protein attachment is the linkage of MT molecules via their N-termini.
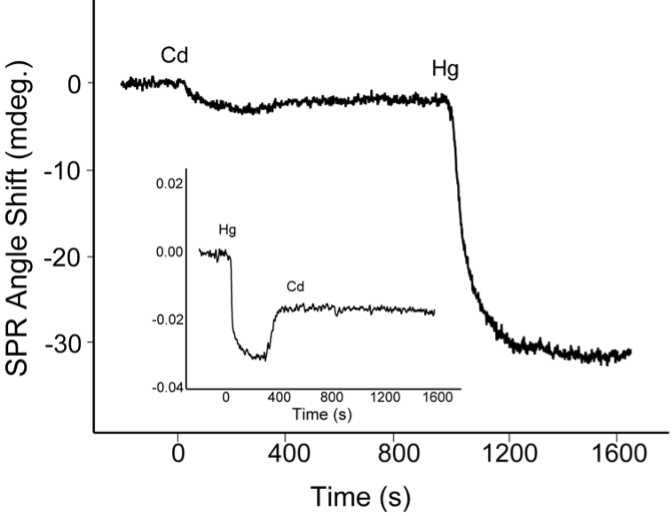
Inset: The apo-MT was first reconstituted with Hg2+ by injecting 100 μM Hg2+ . This was followed by injecting 100 μM Cd2+.
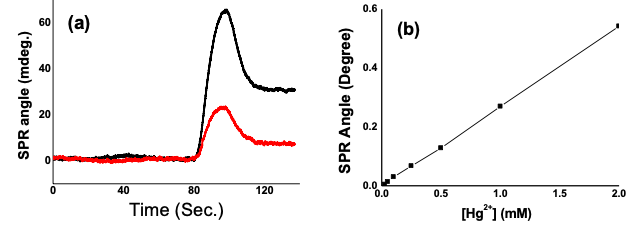
FIG. 3 (a) Time-resolved SPR signals corresponding to the injections of 10 μM (black curve) and 2.5 μM (red curve) Hg2+ into a flow cell housing a hexanedithiol-covered gold sensor chip. (b) Calibration curve constructed by plotting the SPR response as a function of Hg2+ concentration.
Author: Nguyen Ly | Biosensing Instrument | Published May 1, 2010
DOWNLOAD PDF
Download a PDF of Application Note 104: Flow-Injection SPR: Sensitive Determinations of Heavy Metal Ions
- Chah, S.; Yi, J.; Zare, R. N. Sens. Actuators B : Chem. 2004, 99 : 216-222.
- Forzani, E. S.; Zhang, H.; Chen, W.; Tao, N. Environ. Sci. Technol. 2005, 39:1257-1262.
- Zhang, Y.; Xu, M.; Wang, Y.; Toledo, F.; Zhou, F. Sens. Actuators B. 2007, in press.
- Wu, C.-M.; Lin, L.-Y. Biosens. Bioelectron. 2004, 20:864-871.
