Both electrochemical and surface plasmon resonance (SPR) techniques measure various processes taking place at or near an electrode surface. Combining the two techniques allows one to obtain new insight into these interfacial processes. One example is redox-induced conformational changes in surface-bound protein molecules. However, most SPR setups are not suitable for monitoring fast kinetics or small thickness variations. A carefully designed instrument, such as the BI-SPR 1000, is capable of following conformational changes of immobilized proteins (FIG. 1). Boussaad et al.[1] have demonstrated detection of fast redox-induced conformational change in cytochrome c using SPR.
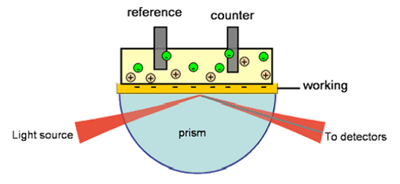
FIG. 1 Redox reaction of a protein is often accompanied by a conformational change. SPR allows one to study surface-confined redox-active proteins.
Redox reactions in proteins and other molecules are known to cause conformational changes in the proteins.[2-5] Such conformational changes are often too small to be monitored using structural analysis techniques, but they can be studied using electrochemical SPR. In the case of cytochrome c, SPR has revealed an apparent thickness change of ~0.04nm [1]. Cytochrome c was immobilized onto a 3-mercaptopropionic acid coated-gold film (SPR sensor chip). Cyclic voltammetry shows a pair of redox peaks near 0.05 V (vs. Ag/AgCl). The coverage of the protein estimated from the cyclic voltammogram is ~6 x 1012 /cm2 (FIG. 2). The simultaneously measured SPR angle shows a sigmoidal change as the protein is switched between the oxidized and the reduced states.
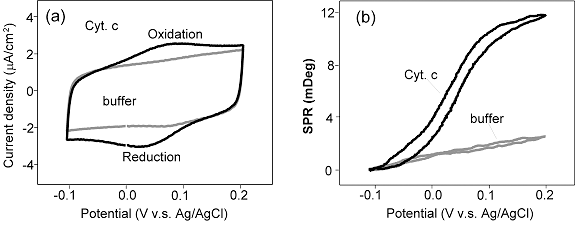
FIG. 2 (a) Cyclic voltammogram of cytochrome c, immobilized on a 3-mercaptopropionic acid-functionalized Au SPR sensor chip and (b) the simultaneously recorded SPR angle shift vs. potential. Note that the cyclic voltammogram and SPR response in the absence of cytochrome c are also shown for comparison.
The SPR shift can be attributed to a conformational change in the protein induced by the redox reaction. This change can alter both the average thickness and the index of refraction of the protein layer which cannot be determined based on SPR shift at a fixed wavelength. But using a simple optical relation, one can estimate that 0.006 deg shift in the dip angle due to the electron transfer corresponds to ~ 0.4 Å change in the thickness. The change is rather small which does not support the conclusion based on hydrodynamic and small angle X-ray measurements but is in good agreement with X-ray crystallography and NMR studies.
Author: Nguyen Ly | Biosensing Instrument | Published April 1, 2009
DOWNLOAD PDF
Download a PDF of Application Note 102: Electrochemical SPR: Redox-Induced Protein Conformational Changes
- S. Boussaad, J. Pean, and N. J. Tao, Anal. Chem., 72:222 (2000).
- C. K. Chan, J. Hofrichter, and A. W. Eaton, Science 274:628.
- W. Colon, A. G. Elove, L. P. Wakem, F. Sherman, and H. Roder, Biochemistry 35:5538 (1996).
- A. M. Berghuis and G. D. Brayer, J. Mol. Biol. 223:959 (1992).
- T. Takano and R. E. Dickerson, J. Mol. Biol. 153:79 (1981).
