IgG deficiencies could be a major reason for impaired humoral immunity, whereas very high IgG levels could be a result of chronic infections, autoimmune disorders, and allergies.1 The levels of IgG in the sera are measured to understand the immune function in patients.2 Clinical assays of total IgG molecules in sera are commonly measured with cellulose acetate membrane electrophoresis3 or immunoturbidimetry4 as they are fast and cost effective. However, the former technique requires highly skilled clinicians because the electropherogram quality is highly dependent on the uniformity of the serum sample and proper handling/maintenance of the membrane, while the latter technique can yield false positive or negative results due to “hook effect”.
Surface plasmon resonance (SPR) is an optical technique capable of accurately determining biomolecular interactions.5-8 Over the years, the sensitivity of SPR has been applied to assessments of affinity, kinetics, and specificity of diverse biomolecular binding interactions, including protein-protein, protein-DNA and DNA-DNA interactions. The rapid determination (RD) SPR method is highly accurate and more sensitive than the two previously mentioned methods for the detection of antibodies from sera as it is label-free and simple to implement and does not need turbidity agents which are required for immunoturbidimetry.
In this study, immunoglobulin (IgG) molecules in sera were accurately and rapidly quantified using BI-4500A SPR system. IgG quantification was done using the initial association phase of their conjugation with His-tagged protein G densely immobilized onto NTA chips, and thereby establishing criteria for selection of the optimal time for constructing the calibration curve as shown in Figure 1.9 The RD SPR method showed high reproducibility (less than 2% RSD) and greater sensitivity than immunoturbidimetry.
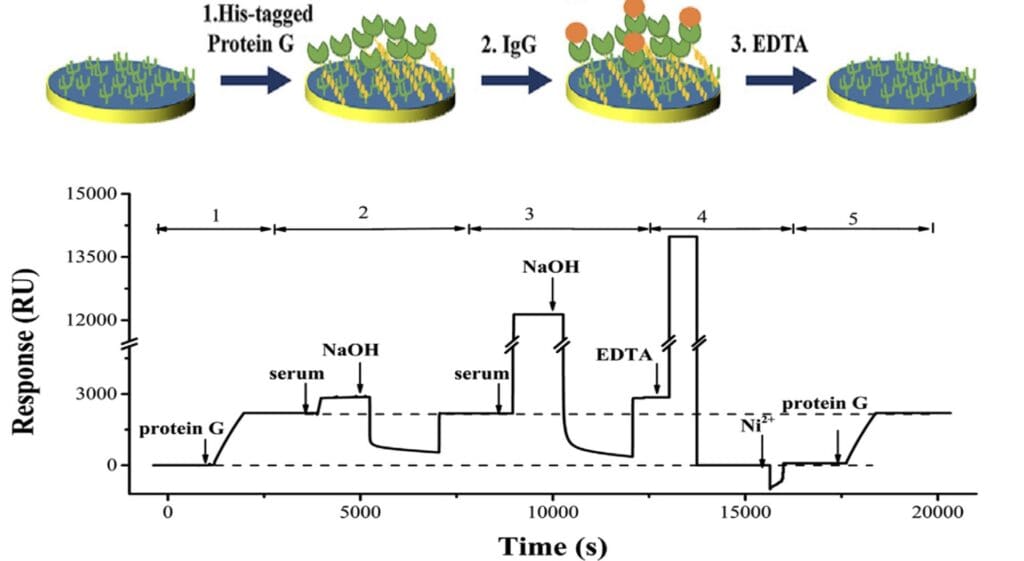
Figure 1: Schematic and SPR sensorgram showing different steps of the IgG analysis in serum samples: (1) immobilization of His-tagged protein G from a 200-nM solution; (2) injection of a 1000- fold diluted serum sample followed by exposing to 20 mM NaOH; (3) injection of a 2-fold diluted serum sample followed by exposing to NaOH; (4) regeneration with EDTA and reloading of Ni 2+; and (5) re-immobilization of protein G of the same density.
The serum samples were drawn from healthy student donors at the University Hospital. The values listed in Table 1 are well within the normal range (5.6 – 17.7 mg mL-1). This is expected as the serum samples were drawn from healthy student donors at the University Hospital. As shown in Table1, the relative differences between the RD SPR method results and those obtained using a commercial immunoturbidimetric kit are all less than 10%. Moreover, a student’s t-test comparing the two sets of data revealed that they are statistically indifferent, on the basis that the calculated t value, 1.67, is lower than both the tabulated t values at the 95% (2.36) and 99% (3.50) confidence levels.10
Table 1: Serum IgG levels measured by BI-4500A SPR system and immunoturbidimetry.
| Samples | SPR (mg mL1 or mg mL-1) | Immunoturbidimetry (mg mL -1) | Relative Differences (%) |
| Serum 1 | 8.72 ± 0.06 | 9.2 | 5.6 |
| Serum 2 | 9.78 ± 0.21 | 9.8 | 0.3 |
| Serum 3 | 11.07 ± 0.11 | 11.9 | 7.5 |
| Serum 4 | 7.72 ± 0.10 | 8.1 | 4.9 |
| Serum 5 | 9.49 ± 0.08 | 9.2 | 3.0 |
| Serum 6 | 9.94 ± 0.07 | 10.8 | 8.6 |
| Serum 7 | 9.88 ± 0.10 | 9.8 | 0.8 |
| Serum 8 | 10.82 ± 0.18 | 10.7 | 1.1 |
The low limit of detection of the RD SPR method used in this study could detect a large IgG signal in a serum sample that had been diluted by 1000 with the running buffer. The original baseline could be readily recovered with NaOH or EDTA injections, which is also effective to renew the chip surface with nickel ions that had been exposed to multiple rounds of serum samples. These observations confirmed that the RD SPR method is ideal for multiple rounds of measurements without the use of anti-fouling materials.
Table 2: Antibody recovery in serum samples.
| Samples | Anti-PSA added (mg mL-1) | Anti-PSA detected (mg mL-1) | Recovery rate (%) |
| 2.50 | 2.53 | 101.2 | |
| Serum | 5 | 4.74 | 94.8 |
| 10 | 9.84 | 98.4 |
Additionally, a sample recovery study by spiking different amounts of anti-PSA (representative IgG antibody) into a serum sample was performed. To determine the recovery rates, the signal from the IgG molecules in the serum was subtracted from the total signal yielded from the spiked serum samples. The recovery rates of anti-PSA spiked into serum samples ranged from 94.8 to 101.2% (Table 2), indicating that the RD SPR method is suitable for the quantification of IgG in human serum without serious matrix effects. The various factors presented in this study would help SPR practitioners to diversify their real-world SPR applications, allowing many more drug candidates to be discovered and assayed.
Author: Nguyen Ly and Miyuki Thirumurthy | Biosensing Instrument | Published April 29nd, 2024
DOWNLOAD PDF
Download a PDF of Application Note 155: Rapid Determination of IgG in Sera using BI-4500A SPR System
- J.A. Freeman, K.R. Crassini, O.G. Best, C.J. Forsyth, N.J. Mackinlay, P. Han, W. Stevenson, S.P. Mulligan, Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia, Leuk. Lymphoma 54 (2013) 99e104
- H.Y. Reynolds, Immunoglobulin G and its function in the human respiratory tract, Mayo Clin. Proc. 63 (1988) 161e174.
- A. Kaplan, J. Savory, W.R. Faulkner, G.G. Rudolph, W.J. Ford, M.E. Deutsch, Cellulose acetate electrophoresis of proteins of serum, cerebrospinal fluid, and L. Liu, C. Han, M. Jiang et al. Analytica Chimica Acta 1170 (2021) 338625 8 urine, in: R.P. MacDonald (Ed.), Standard Methods of Clinical Chemistry, Elsevier, 1970, pp. 13e30.
- E. Denham, B. Mohn, L. Tucker, A. Lun, P. Cleave, D.R. Boswell, Evaluation of immunoturbidimetric specific protein methods using the Architect ci8200: comparison with immunonephelometry, Ann. Clin. Biochem. 44 (2007) 529e536.
- L. Wu, Y. He, Y. Hu, H. Lu, Z. Cao, X. Yi, J. Wang, Real-time surface plasmon resonance monitoring of site-specific phosphorylation of p53 protein and its interaction with MDM2 protein, Analyst 144 (2019) 6033e6040.
- H. Zhao, I.I. Gorshkova, G.L. Fu, P. Schuck, A comparison of binding surfaces for SPR biosensing using an antibody-antigen system and affinity distribution analysis, Methods 59 (2013) 328e335.
- Y. He, Z. Wang, Y. Hu, X. Yi, L. Wu, Z. Cao, J. Wang, Sensitive and selective monitoring of the DNA damage-induced intracellular p21 protein and unraveling the role of the p21 protein in DNA repair and cell apoptosis by surface plasmon resonance, Analyst 145 (2020) 3697e3704.
- H. Zhang, L. Yang, B. Zhou, X. Wang, G. Liu, W. Liu, P. Wang, Investigation of biological cell-protein interactions using SPR sensor through laser scanning confocal imaging-surface plasmon resonance system, Spectrochim. Acta Mol. Biomol. Spectrosc. 121 (2014) 381e386.
- Liu, L., Han, C., Jiang, M., Zhang, T., Kang, Q., Wang, X., Wang, P. and Zhou, F., 2021. Rapid and regenerable surface plasmon resonance determinations of biomarker concentration and biomolecular interaction based on tris-nitrilotriacetic acid chips. Analytica Chimica Acta, 1170, p.338625.
- D.C. Harris, Quantitative Chemical Analysis, eighth ed., W. H. Freeman and Company, New York, U. S. A., 2010.
