Surface Plasmon Resonance (SPR) has emerged as a sensitive, real-time and label free technique for quantifying biomolecular interactions.[1] By combining SPR with electrochemical methods, many new capabilities and applications are now possible. This application note describes a recent study of liver cancer cells with the electrochemical SPR module developed by Biosensing Instrument.

Figure 1 Schematic of EC SPR live cancer cells experiment after treatment of DNR
The study was carried by Wu et al [2] using a BI-2000 SPR with EC analysis module and an electrochemical workstation (660B CH) (Fig. 1) to measure apoptosis of cancer cells (HepG2) after treatment of Daunorubicin (DNR), an anti-cancer drug commonly used to treat specific types of leukemia. DNR was freshly prepared and stored in the dark at 4 °C. HepG2 cells were cultured in DMEM supplemented with 10% fetal bovine serum at 37 °C with 5% CO2 and 95% humidified atmosphere. The SPR chips were placed into the wells of a six-well plate and then rinsed with the cell culture medium. Cells with different concentrations were added to the wells and then incubated for 2, 4, and 6 h, respectively, before the electrochemical SPR measurement. DNR solutions of different concentrations were injected into the BI electrochemical analysis module using a pipette, and SPR angle changes and cyclic voltammetry data were simultaneously recorded. The potential was cycled between -0.8V and 0.8V at the rate of 0.1 V s-1.
Fig 2 is the cyclic voltammograms (CV) of DNR (10 uM) with and without HepG2 cells in the medium. Without HepG2, the CV (red) shows a pronounced peak at ~0.6 V. The peak shifts positively after incubating with the HpeG2 cells (blue). However, in the absence of DNR, the CV does not show this peak. The redox activity was attributed to two electroacive groups (a p-diquinone and a p-diphenol) of DNR. [3]
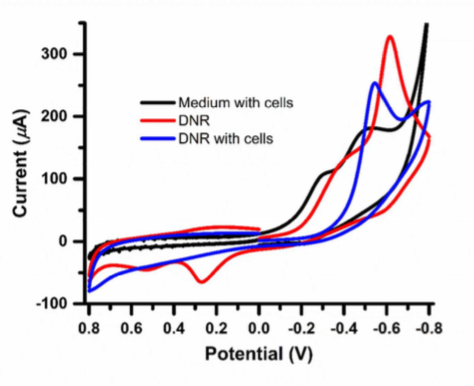
Figure 2 Cyclic voltrammograms of HepG2 cells, DNR (10 UM) with and without HepG2 cells.
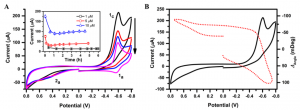
The redox peak decreased with time and to a level 50% 1 h after treating the cells with DNR (Fig. 3A). Incubation of the cells with DNR led to apoptosis of the cells, eventually detachment from the SPR chip surface, which exposed the Au film to the cell culture medium, and thus increased the electrochemical current. A further study of current change vs. DNR concentration and incubation times was performed. The two oxidation peaks, 1a and 2a, showed up after the second cycle of potential scan (1a could be attributed to the reduced product of DNR adsorbed on the SPR chip). The SPR signal decreased as the potential cycled to negative direction (Fig 3B) due to the removal of oxidized layer on the Au electrode.[4]
In summary, DNR treatment of HepG2 cells changes the morphology and mass distribution of the cells, which can be monitored with SPR. It also leads to apoptosis, which can be simultaneously studied with electrochemical and SPR measurements. This study demonstrates a unique application of combined electrochemical and SPR techniques.
Author: Nguyen Ly | Biosensing Instrument | Published May 4, 2020
DOWNLOAD PDF
Download a PDF of Application Note 120: Electrochemical SPR Monitoring of Drug Interactions with Live Cancer Cells
- Schasfoort, Richard B M and Tudos, Anna (eds), RSC Publishing, 2008, 1-14
- Wu, C et al, ACS Appl. Mater. Interfaces 2015, 7, 24848-24854
- Jiang, H and Wang, X, Electrochem. Commun., 2009, 11, 126-129
- Lioubimov, V et al, Appl. Opt. 2004, 43, 3426-3432