This application note describes the simultaneous SPR detection of wild-type and mutant p53 proteins in cancer cell laysates. p53 is a transcription factor (i.e., DNA-binding protein) that plays an important role in DNA repair and tumor suppression by inhibiting the growth of tumor cells through eliciting either cell-cycle arrest or apoptosis [1-3]. In solution, p53 molecules tetramerize at their C-termini and the resultant p53 tetramer can bind to the double-stranded (ds)-DNA containing two or more copies of the consensus sequence [1-3]. The mutation of the p53 gene has been linked to more than 50% of cancer cases. The consequence of the p53 mutation is the loss of its ability to bind consensus ds-DNA, which has the sequence of PuPuPuC(A/T)(T/A)GPyPyPy (where Pu and Py represent purines and pyrimidines, respectively). Clinically it is important to determine the extent of p53 mutation (i.e., the ratio of wild-type p53 over the mutant counterpart) and gauge the alteration of its binding affinity to consensus ds-DNA.
Fig. 1 depicts the scheme developed by Wang et al.[4] for simultaneous SPR detections of wild-type and mutant p53 proteins. A carboxymethylated dextran film was first coated onto a Au sensor chip. When the chip is mounted onto the BI-SPR instrument, between the chip and the PDMS gasket two fluidic channels are formed, into which different solutions can be introduced through the external valving system. In the present application, an anti-p53 antibody (PAb421) which can capture both wild-type and mutant p53 molecules, was covalently attached to the chip surface confined by Channel 1. Consensus ds-DNA was affixed inside Channel 2 via covalent linkage of aminated single-stranded (ss)-DNA followed by hybridization with its complementary DNA strand. When samples are injected to these fluidic channels, both mutant and wild-type p53, represented by the blue and red diamonds in Fig. 1, respectively, are recognized by the antibody. However, the consensus ds-DNA will only interact with the tetramer of the wild-type p53 (cf. Channel 2 depicted in Fig. 1). Therefore, the difference in the SPR responses between the two fluidic channels represents the extent of p53 mutation.
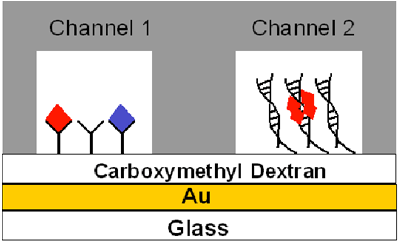
FIG. 1 Schematic diagram showing the principle behind simultaneous detection of wild-type and mutant p53 molecules in the dual channel flow cell of the BI-SPR. The gray area represents the PDMS gasket pressed onto the Au sensor chip to form two rectangular fluidic channels.
Table 1 shows the concentrations of wild-type p53, total p53, mutant p53, and the extent of mutation determined from two cancer cell lines and two normal cell lines. The concentrations are deduced by comparing the SPR signals to calibration curves constructed from measuring a series of p53 standard solutions (see Ref. [4] for more details). As can be seen, the extent of mutation in the cancer cell lysates is significantly greater. From this study, it is apparent that the use of the dual-channel BI-SPR affords a simple, selective, and accurate method for the simultaneous detection of mutant and wild-type p53 in cancer cell lysates.[4]
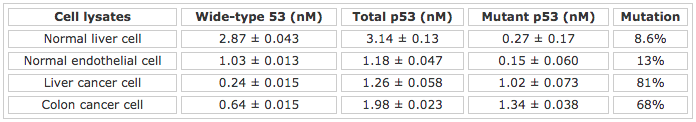
Table 1 Detection of p53 in normal and cancer cell lysates
The standard deviations were computed from three replicate measurements
Author: Nguyen Ly | Biosensing Instrument | Published May 4, 2020
DOWNLOAD PDF
Download a PDF of Application Note: 112 – Detection of Wild-Type and Mutant p53 Proteins in Cancer Cell Lysates
- Vogelstein, B.; Lane, D.; Levine, A. J. Nature 2000, 408, 307-310
- Ko, L. J.; Prives, C. Genes Dev. 1996, 10, 1054-1072
- Weintraub, H.; Hauschka, S.; Tapscott, S. J. Proc. Natl. Acad. Sci. USA 1991, 88, 4570-4571
- Wang, Y.; Zhu, X.; Wu, M.; Xia, N.; Wang, J. Zhou, F. Anal. Chem. 2009, 81, 8441-8446
