A major advantage of SPR technology is that the detection does not require the analyte to be labeled, such as with a fluorescent molecule or a redox-active tag. BI-4500 is a powerful instrument providing detailed label-free binding kinetics for a wide range of molecular interactions. This is because SPR directly detects changes in refractive index resulting from changes in mass at the sensor chip surface. For researchers interested in pharmacology and pharmacokinetics or in general pharmaceutical research or drug discovery, this capability of label-free detection is particularly attractive.
Carbonic anhydrase II (CAII), which is an enzyme that rapidly converts carbon dioxide and water to bicarbonate (with proton as a co-product). Carbonic anhydrase inhibitors are a class of pharmaceuticals that inhibit the activity of carbonic anhydrases.1 Clinically, these inhibitors have been used as antiglaucoma agents to alleviate mountain sickness and to manage neurological disorders. This application note presents the SPR binding experiment between CAII and small molecule inhibitors like Acetazolamide (222.22 Da), 4-Carboxybenzene sulfonamide (201.2 Da), Sulphanilamide (172.2 Da) and Sulpiride (341.4 Da) using highly sensitive carboxyl sensors. The binding rate constants and affinity value obtained from the kinetics analysis (red lines) of the SPR binding curves (black or blue lines) are shown in Figure 1 and Table 1.

Figure 1: Sensorgrams of acetazolamide, 4-carboxybenzene sulfonamide, sulphanilamide and sulpiride interacting with a 5000-RU CAII surface. The highest concentration for each compound was as follows: (A) acetazolamide, 10 uM; B) carboxybenzenesulfonamide, 40 uM; (C) sulfanilamide, 80 uM; (D) sulpiride, 1000 uM. Each compound was injected using a 3-fold dilution series. The entire data set was fit globally with a 1:1 interaction model as shown by red lines.
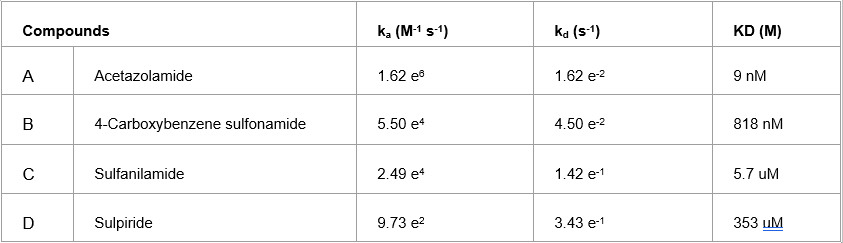
Table 1: Kinetic values of small molecule binding to CAII.
Excellent fits were achieved and the kinetic and binding parameters in the table are in good agreement with published results.2&3 The feasibility for small molecule detection across a wide affinity range can be attributed to the high sensitivity and low noise of BI SPR detection technology, the fine precision and stability of its BI-DirectFlow™ sampler delivery system, and the excellent performance of BI sensor chips having high loading capacity with low non-specific adsorption as seen in Figure 2.
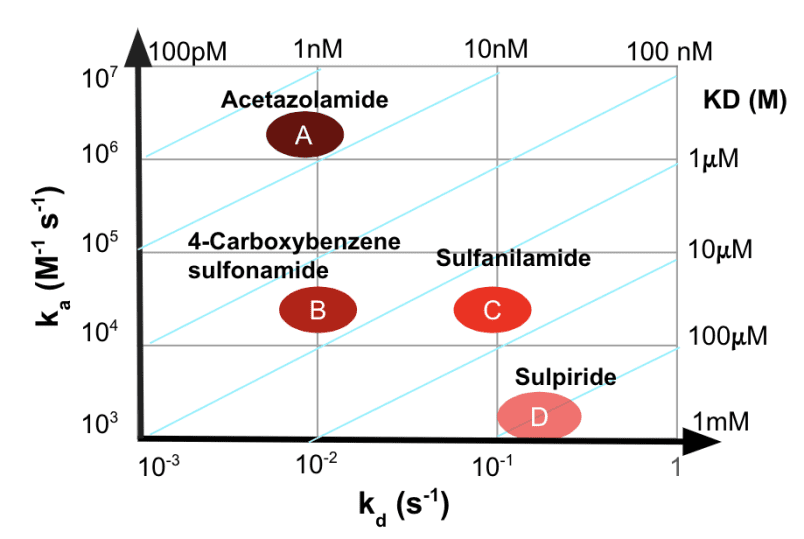
Figure 2: Isoaffinity plot of all the four compounds. The association rate constants are on the y-axis. The dissociation rate constants are on the x-axis. The intersection of the ka and kd gives an equilibrium dissociation constant.
This note demonstrates that SPR can be used to study the binding interactions of small molecules to proteins. SPR for small molecule detection is a powerful technique that can be used in applications such as screening drug candidates to combat cancers, alleviate the symptom of neurological disorders, and discover antifungal and antibacterial agents.
Author: Nguyen Ly | Biosensing Instrument | Published May 4, 2020
DOWNLOAD PDF
Download a PDF of Application Note 119: Small Molecule Detection by Surface Plasmon Resonance (SPR)
- Supuran CT, Scozzafava A, Casini A (March 2003). "Carbonic anhydrase inhibitors". Med Res Rev 23 (2): 146–89
- Cannon, M. J., Papalia, G. A., Navratilova, I., Fisher, R. J., Roberts, L. R., Worthy, K. M and Myszka, D. G. (2004). Comparative analyses of a small molecule/enzyme interaction by multiple users of Biacore technology. Analytical biochemistry, 330(1), 98-113.
- Myszka, David G. "Analysis of small-molecule interactions using Biacore S51 technology." Analytical biochemistry 329.2 (2004): 316-323.
