Electrochemical impedance spectroscopy (EIS) is a technique traditionally used in corrosion monitoring, coatings evaluation, batteries, and electrodeposition and material characterization. In recent years, this technique has gained momentum in biotechnology, tissue engineering, and characterization of biological cells and disease diagnosis for the monitoring of cell adhesion, proliferation, differentiation, apoptosis, and degenerative processes. (1,2)
Electrochemical impedance can be measured through the response of an electrochemical system to an applied potential and the frequency dependence of this impedance can reveal underlying chemical processes. In a typical EIS configuration, the stimulus signal (AC voltage, delta V) is applied between the working electrode (WE) and reference electrode (RE), while the current response (delta I) is measured at the counter electrode (CE). Impedance Z (Z= delta V/delta I) is very sensitive to the properties of the electrode surface and to molecular adsorption taking place on the electrode surface, which is the basis of impedance spectroscopy to study interfacial phenomena and biosensor applications. (3)
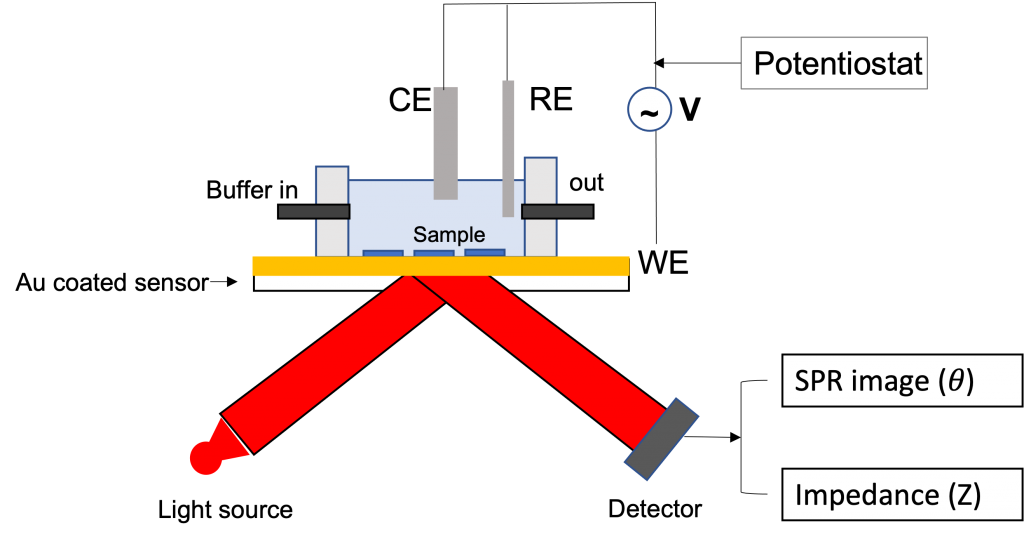
Surface Plasmon Resonance microscopy (SPRm) Impedance measures both SPR and EIS simultaneously and is capable of imaging local electrochemical impedance in real time with high spatial resolution. This technique relies on detecting surface plasmon waves propagating on a metal thin film which are created by a laser beam incident on the metal film, typically via a prism at a resonant angle. In the same way as for EIS, an AC voltage is applied to the surface to create a surface charge density modulation (delta q), which induces a SPR signal change (delta theta). The surface plasmon resonance condition is sensitive to the dielectric constant of the metal film, which is a function of the surface charge density. The charge density q of a surface is related to the SPR signal theta by
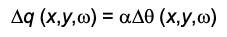
where omega is the angular frequency of the AC modulation and x,y denote a location on the electrode. alpha ≈ 28 C m-2 deg-1 is a constant that can be determined either by experimental calibration or calculation.
From the surface charge density distribution, the local impedance Z of the surface is represented by
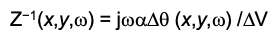
where j = square root -1 and delta V is the amplitude of the applied voltage modulation.
This equation arises from the fact that current density J is the rate of change of the surface charge density, given by
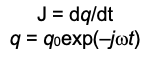
where q0 is the amplitude of the surface charge density modulation induced by the voltage modulation. (4)
Compared to SPR, the impedance signal measured by SPRm Impedance is insensitive to nonspecific binding and solution bulk index changes and because the signal scales with the surface area, it is suitable for detecting small molecules. (5)
In conclusion, by measuring SPR in an electrolyte, interfacial impedance can be measured by applying a potential modulation to the sensor surface. By measuring the amplitude and phase of the resonance angle produced by the changes in the surface charge modulation, submicron spatial resolution impedance information can be obtained to study the cellular responses dynamics or to study electrochemical reactions of nanoparticles.
References:
(1) Isaac O. K’Owino et al, Electroanalysis 2005, 17, 23, 2101-2113
(2) Katarzyna Krukiewicz, Electrochemistry Communications 2020, 116, 106742
(3) Kyle Foley et al, Anal Chem 2008, 80, 13, 5146-5151
(4) Wei Wang et al, Nat Chem 2011, 3, 249–255
(5) Wenbin Liang et al, Anal Chem 2014, 86, 9860-9865
