Radioligand binding assay has been used to measure the affinity of ligand binding to membrane protein receptors, including G-protein coupled receptor (GPCR). The first radioligand binding assay was performed in the 1960s. Since then, better receptor preparations, more radiolabeled ligands and higher radioactivity have been developed, thus, making the radioligand binding method an important tool in the development of drugs in the pharmaceutical industry. The basic principle of radioligand binding assay is the thermodynamic equilibrium of ligand-receptor interactions, involving association and dissociation, given by
(1) ![]()
At equilibrium, the rate of association equals the rate of dissociation, and the equilibrium constant, is given by
(2) 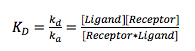
where ka and kd are the association and dissociation constants, respectively. measures the concentration at which half the receptors are occupied by the ligands, and ka and kd quantify the association and dissociation kinetics.
The radioligand binding assay measures the equilibrium dissociation constant (KD), but it cannot directly measure association (ka) and dissociation (kd) kinetics. Surface Plasmon Resonance (SPR) is a widely used label-free detection technique for measuring both the equilibrium dissociation constant, and the kinetic constants. Since its commercialization in 1990s, SPR has become a central tool in biomedical research, biosensor development and drug discovery. However, studying membrane proteins by the traditional SPR techniques is challenging mainly due to overall yield of protein after purification and the poor protein stability under detergents and lipids working environment. For these reasons, only a very few examples SPR study of membrane proteins have been reported.
SPR microscopy (SPRM) is a new technique that combines high-resolution optical microscopy with SPR technology. A unique advantage of SPRM is its capability to directly measure both equilibrium binding affinity and kinetics of ligand-membrane protein interactions, such as the binding of ligands with GCPRs. Furthermore, SPRM is a label-free technique so there is no need to use radioactive isotopes for the experimental assay.
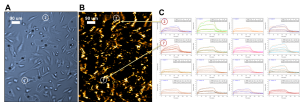
In SPRM, cells are incubated or immobilized on the sensor surface, and the ligand (drug molecules) are delivered to the sensing area to perform the binding measurement. Figure 1 shows results of an interaction between a small molecule drug and GPCR target (on overexpressed CHO cells). The real-time SPR image (1B, dark brown image) indicates the interactions or binding response (gold area) of cell receptors to the drug molecules. By selecting the areas of interest based on a bright field image (1A, blue image), sensorgrams can be generated (1C) and binding information (KD, ka and kd) can be derived. When many different regions of cells are chosen, the heterogeneity of the cell and cell membrane can be determined with statistically meaningful data. Due to the excellent SPR resolution, the distribution and local binding activities of membrane proteins in each cell can be determined and mapped.
The table below summarizes the pros and cons of radioligand binding assays and SPR microscopy.
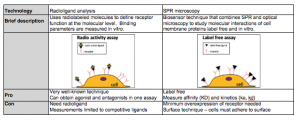
In summary, radioligand binding assay is a commonly used technique to study ligand binding to membrane proteins, such as GPCRs. Its main limitations are the need of radioactive isotope labeling, lack of direct kinetic information and lack of single cell resolution. SPRM is a new technology that can directly measure the equilibrium and kinetic constants of ligand-membrane protein interactions on cells without labeling.
Authors: Nguyen Ly | Biosensing Instrument | Published Dec 1, 2018
DOWNLOAD PDF
Download a PDF of Technical Note 106: SPR Microscopy vs Radioligand Binding Analysis