Millions of sepsis-related deaths are reported every year, which makes acute septic shock one of the leading causes of death in intensive care units around the world.1 Septic shock is characterized by multiple organ failure, which is generally initiated by the activation of alpha-2A adrenergic receptor (α2A adrenoceptor, ADRA2A) in Kupffer cells.2&3 These effects can be reversed upon infusion of ADRA2 antagonists such as yohimbine (nonselective) or BRL-44408 maleate (ADRA2A selective).4 Furthermore, yohimbine has demonstrated significant anti-inflammatory and antifibrotic activity in both in vitro (hepatic endothelial and stellate cells and hepatocytes) and in vivo (hepatic inflammation/fibrosis) models.5
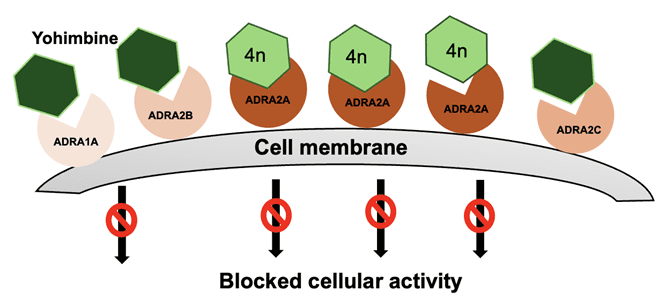
Figure 1: Schematic of Yohimbine and 4n derivatives binding to various ADRA receptors. 4n binds more effectively to the ADRA2A compared to other derivatives.
Yohimbine is a naturally occurring indole alkaloid found in plants which exhibits affinity to several types of receptors, among which the highest affinity is toward ADRA2 receptors. However, a variety of side effects such as hypertension, nausea, gastrointestinal issues, and serotonin syndrome, along with the low selectivity of yohimbine towards ADRA2 receptor represent a substantial obstacle for the application of yohimbine in clinical use.6 Till date, there is very limited information about the selective inhibition mechanism of parent yohimbine and its derivatives towards individual ADRA2 receptor subtypes. Therefore, identifying a yohimbine analogue with improved properties as a potential preclinical candidate for treatment of sepsis and liver inflammation would be of great benefit.
In this study, researchers from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences employed computational simulation together with biophysical assays such as Surface Plasmon Resonance Microscopy (SPRM) to identify new yohimbine derivatives exhibiting high antagonist potency against ADRA2A adrenergic receptor with improved selectivity over related off-target receptors (Figure 1).7 This research addresses treatment of noradrenaline-mediated peripheral inflammatory disorders such as septic shock and organ failure.
A structure-activity relationship study of the yohimbine analogues identified that the synthesized derivatives 4g, 4l, 4m, and 4n had at least 5 times higher ADRA1A/ADRA2A selectivity index compared to the parent yohimbine. Moreover, the derivative 4n had 25 times higher selectivity to ADRA2A receptor over ADRA2B as compared to the other yohimbine, according to the endpoint analysis assays. Also, compound 4n demonstrated high plasma and microsomal stability, moderate-to-low membrane permeability, and negligible toxicity on nontumor normal human dermal fibroblasts. However, the direct binding interaction between the yohimbine and its structural derivatives with ADRA1A/ADRA2A receptors in the native cellular environment have not been studied using in vitro assays thus far.
For measurement of real-time binding activities of yohimbine and 4n directly on ADRA2A overexpressing Chem-1 cells, label-free cell-based SPRm 200 instrument was used. The cells were seeded on a gold sensor chip with a silicone well for 24 h before the experiment at a concentration of 80,000 cells per well in a standard medium. The cells were treated with the following concentrations: 0.14, 0.41, 1.2, 3.7, 11.1, 30, 100, 300, 1000 and 3000 nM for both yohimbine and 4n. The ImageSPRTM software was used to analyze the data, and KD values obtained from SPRM on live cells were 8.1 nM for Yohimbine and 28.2 nM for 4n as shown in Figure 2. The equilibrium constant (KD) calculated for yohimbine and for lead compound 4n using SPRm 200 on live cells matched the KDs obtained from the end point functional assays (calcium flux assays and radioligand binding assay). SPRM has the unprecedented advantage of measuring binding kinetics on whole cells which is critical to thoroughly assess pharmacological efficacy, biorelevant pharmacokinetics, and fundamental cellular processes essential for drug development.
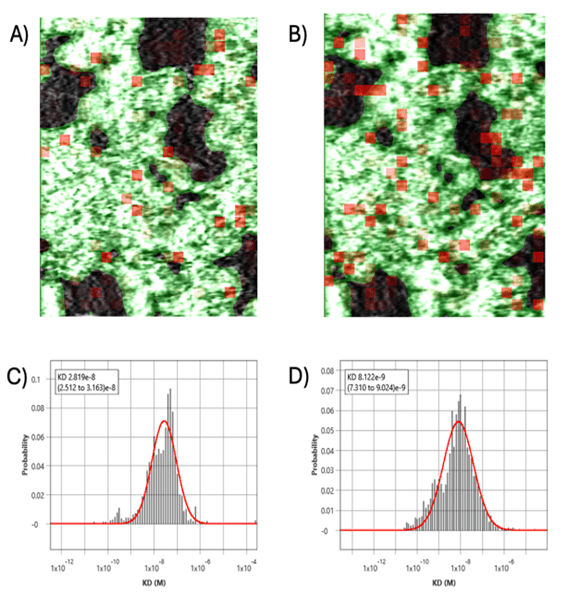
Figure 2: Binding Data of (A)4n binding to ADRA2A overexpressing Chem-1 cells and (B) Yohimbine bound to Chem-1 cells; ROI squares on SPR images indicate areas where binding events were detected. The kinetic binding parameters extracted from every ROI were aggregated to form KD histograms for 4n (C) and Yohimbine (D).
SPRM confirms the direct interaction of 4n with the ADRA2A receptor in real-time using whole cells and reveals the binding selectivity of 4n in the live cell environment, yielding biologically relevant information for the first time. Additionally, this straight forward, simple, label free, real-time binding SPRM assay has shown that the compound 4n represents a novel pharmacological probe to investigate various adrenoceptors, and that it is a promising lead candidate for further preclinical development to treat sepsis-induced liver inflammation and other ADRA2A receptor-mediated pathologies.
Author: Nguyen Ly, and Miyuki Thirumurthy | Biosensing Instrument | Published February 10th, 2025
DOWNLOAD PDF
Download a PDF of Application Note 163: Binding Activities of Yohimbine Analogues on ADRA2A Overexpressing Live Cells
- Fleischmann, et al, American journal of respiratory and critical care medicine 193.3 (2016): 259-272.
- Wang, P et al, J. Physiol.: Regul., Integr. Comp. Physiol. 1996, 270 (5), R927.
- Zhou, M et al, Biochim. Biophys. Acta, Mol. Basis Dis. 2005, 1740 (3), 446– 452.
- Miksa, M et al. PLoS One 2009, 4 (5), e5504.
- Sharma,N et al, Phytomedicine 2024, 123, 155182 DOI: 10.1016/j.phymed.2023.155182.
- Philipp, M et al, J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283 (2).
- Chayka, et al. Journal of Medicinal Chemistry (2024).
