In this application note, binding interactions of an antibody to a membrane transport receptor were studied using SPRm 200. Transporters have important roles in physiological processes ranging from cellular uptake of nutrients to the absorption of drugs.(1) Despite their importance as a drug discovery, it is difficult to determine its function: direct biophysical studies require these proteins be solubilized and purified and between their extraction and reconstitution, transport activity cannot be measured because of the lack of a vectorial environment.(2)
SPR microscopy (SPRM) is a new technique that combines high-resolution optical microscopy with SPR technology. A unique advantage of SPRM is its capability to directly measure both binding affinity and kinetics of ligand-membrane protein interactions without extracting the proteins from the cell membrane, thus ensuring measurements in the membrane’s physiological environment.(3) The bright field optics of the instrument provides topographical and phenotypical responses of the cells to a ligand (drug molecule), and the SPR measures, simultaneously, the binding kinetics of the ligand to the receptors expressed on the cell surfaces.
Target receptors were expressed on HEK293 cells grown on SPRm chips, following standard SPRm cell preparation protocols. Using a custom running buffer, 5 concentrations of antibody were injected to each chip using the kinetic titration method. The experiment generated KD= 2.9 nM (2.0 – 4.3 95% CI), kon= 2.1 x 105 M-1 s-1 (1.6 to 2.9 95% CI) and koff= 7.4 x 10-4 s-1 (5.7 to 9.6 95% CI). The KD is consistent to functional assay results. It is the first time we know of that the kinetic constants (ka, kd) have been measured for membrane transport proteins in a label-free manner.
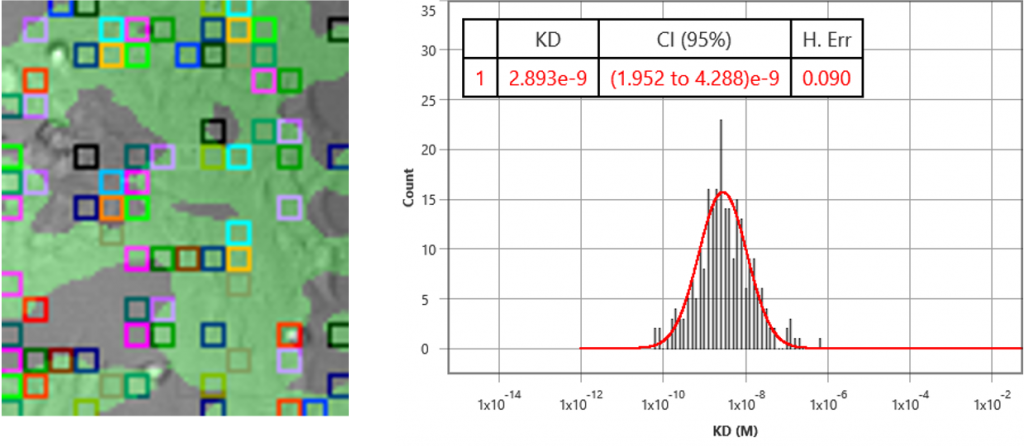
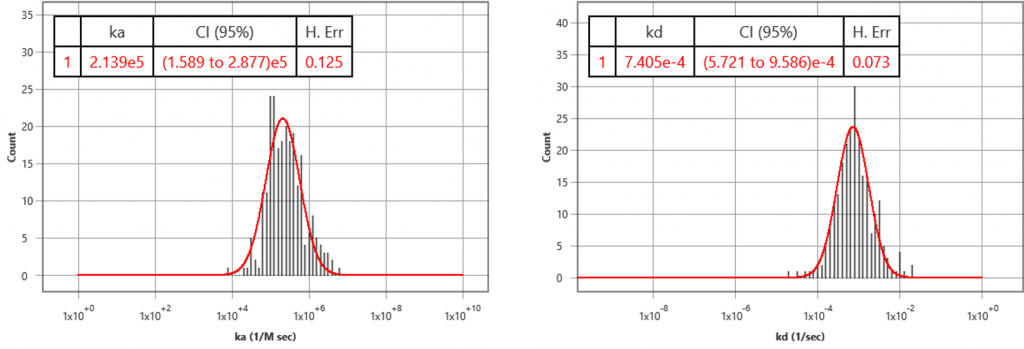
In summary, SPRM is a powerful technology that measures binding affinity and kinetic constants of membrane targets which are difficult to obtain using traditional labelled and label-free methods. Measuring biophysical properties in the membrane target’s native environment in real time and label free provides insightful drug-receptor interactions information much earlier in the drug discovery process, thus opening an opportunity to speed up drug development.
[application_pdf] [note_references]