Glycogen is the primary storage form of glucose. Glycogen synthesis and breakdown are tightly controlled by glycogen synthase (GYS) and phosphorylase, respectively. The enzyme responsible for the process is protein phosphatase 1 (PP1). The dephosphorylation of glycogen synthase and phosphorylase a (glycogen phosphorylase phosphorylated on Ser14 is commonly referred to as phosphorylase a) by PP1 will cause the activation of glycogen synthase and the inactivation of phosphorylase a, consequently the regulation of glycogen synthesis. (1,2)
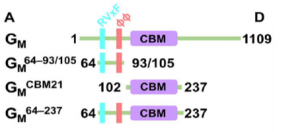
The ability of PP1 to regulate glycogen synthesis also requires its association with a specific regulatory subunit, the glycogen-targeting subunit in muscle, GM. GM is a 1109-amino acid protein (124 kDa). It was previously shown that residues 1 to 240 are required for PP1 binding and regulation. Bioinformatics predicts that GM residues 1 to 100 are intrinsically disordered, while residues102 to 237 form a well-folded domain that is a member of the CBM21 family. Residues 64 to 93/105 include a highly conserved RVxF motif, which is essential for PP1 binding, and an extended ΦΦ motif. GM CBM21 is known for glycogen binding, how ever it is unclear whether it contributes directly to PP1 biding or not. In this experiment, a five channel SPR, BI-4500, was used to help identify the binding sites of GM to PP1.(3)
Isothermal titration calorimetry (ITC) showed that GM residues 2 to 64 do not bind PP1, as both GM 2–237 and GM 64–237 bind PP1 with statistically identical affinities (KD) values of 27 and 21 nM, respectively. It is also noticeable that GM 64-237 includes both the RVxF and the CBM21 sites. With the ITC data along, it was unable to distinguish the role of the two, because of difficulty in obtaining binding data between PP1 and GM CBM21.
To solve the question, SPR was used to measure the binding kinetics between GM 64– 105 and PP1. His6-tagged PP1a7–330 (62.5 nM) in 20 mM tris (pH 8.0), 500 mM NaCl, 0.5 mM TCEP, 1 mM MnCl2, and 0.005% Tween-20 was loaded onto a Ni2+-NTA chip (Biosensing Instrument Inc.) using different loading times (20, 40, 60, and 80 s) to achieve different PP1 densities on the His6-sensor chip in four different channels (channel 1 was the reference channel). GM 64–105 was prepared in the same buffer as PP1a7–330 using 1:3 serial dilutions (31.25 to 500 nM). Results from the SPR measurements are shown in Figure 2 below.
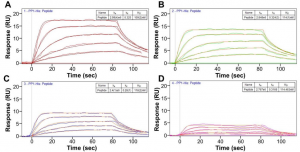
FIG. 2 Surface plasmon resonance analysis of GM 64-105 : PP1α7-330. Sensograms of GM 64-105 (analyte) binding to His6-tagged PP1α7-330 immobilized on a Ni+2-NTA chip (Biosensing Instruments). Panels (A-D) correspond to different amounts of His6-tagged PP1 immobilized on the chip. Sensograms show different response units (RU) as a function of varying concentrations of GM 64-105 (0 nM, 31.25 nM, 62.5 nM, 125 nM, 250 nM and 500 nM). Global fit to a 1:1 binding model is shown.
With SPR, it was shown that GM 64–105 binds PP1a7–330 with a KD of 114 ± 4 nM, revealing that GM CBM21 directly contributes to PP1 binding (~4-fold increase in PP1 binding with GM 64–237 versus GM 64–105) and thus PP1 and GM bind one another via two distinct interaction sites.
In conclusion, highly sensitive SPR measurements were able to help in identifying that PP1 interacts with GM outside its RVxF sequence via the extended ΦΦ motif and the GM CBM21 domain, which results in extremely tight binding.
Materials extracted from Dr. W. Peti’s publication in Ref. 3. SPR data was measured with the help from Biosensing Instruments researchers.
Author: Nguyen Ly | Biosensing Instrument | Published Jan 4, 2025
DOWNLOAD PDF
Download a PDF of Application Note 133: Interaction of Protein Phosphatase 1 with its Muscle Glycogen– targeting Regulatory Subunit Measured by SPR
- P. Dent, A. Lavoinne, S. Nakielny, F. B. Caudwell, P. Watt, P. Cohen, The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature 348, 302–308 (1990).
- J. Liu, D. L. Brautigan, Glycogen synthase association with the striated muscle glycogen targeting subunit of protein phosphatase- 1. Synthase activation involves scaffolding regulated by b-adrenergic signaling. J. Biol. Chem. 275, 26074–26081 (2000).
- G. Kumar, M. S. Choy, D. M. Koveal, M. K. Lorinsky, S. P. Lyons, A. N. Kettenbach, R. Page, W. Peti1, Identification of the substrate recruitment mechanism of the muscle glycogen protein phosphatase 1 holoenzyme, Sci. Adv. 2018;4: eaau6044 14 November 2018.