G-protein coupled receptors (GPCR) are integral membrane proteins that transmit signals from external stimuli to the cell interior. They play key roles in many cellular processes, such as sensorial, hormonal, metabolic, immunological, and neurotransmission processes(1), and are the most popular membrane protein drug targets for a wide range of diseases, including cancer, immune and inflammatory disorders as well as neurological and metabolic diseases. (2, 3) Despite the importance, measuring and quantifying the binding of ligands or drug candidates with GPCRs are extremely difficult with traditional detection technologies.
In this application note, we describe a study of the binding of histamine to GPCR in vitro on the surface of intact cells with SPRm 200, a label-free SPR Microscope. This technology enables direct measurement of binding kinetics of membrane proteins, e.g., GPCRs, on single cells without the need of extracting them from the cells. With this system, the individual cells are visualized with the bright field optical microscopy, and binding is quantified in real time simultaneously with the SPR.
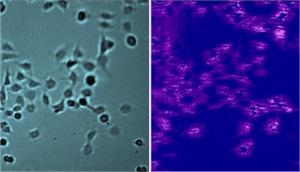
Human embryonic kidney cells 293 (HEK 293A) with overexpressed histamine GPCRs were incubated on the SPR sensor surface and fixed with 4% PFA (paraformaldehyde) at room temperature. Binding of the 500 Da compound at five different concentrations were measured for multiple cells. While KD for different cells varied from 2.3 nM to 9.4 nM, the average KD was determined to be 7.41 nM (Fig 1), which is consistent with published values. (4, 5)
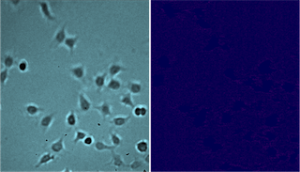
As a negative control, HEK 293A cells with the mutated histamine GPCRs unable to bind histamine were also studied. As expected, no binding was detected (Fig 2).
In conclusion, SPRm 200 is a new and powerful technique to study GPCR binding affinity directly on the whole cells in a label-free manner. With SPRm 200, quantitative mapping of binding affinity of multiple cells can be observed in real time.
Author: Nguyen Ly | Biosensing Instrument | Published Jan 4, 2025
DOWNLOAD PDF
Download a PDF of Application Note 126: GPCR Binding Assays with SPR Microscopy
- Locatelli-Hoops, S et al, Biomed Spectrosc Imaging, 2(3), 155-181, 2013
- Szafran K et al, Pharmacol Rep, 65(6), 1498-505, 2013
- Hutchings, C et al, Nature Reviews Drug Discovery, 16, 1-24, 2017
- del Ouvillo, A et al, J Investig Allergol Clin Immunol 16, 1:3-12, 2006
- Shahid, M et al, The Open Immunology Journal, 2, 9-41, 2009