Membrane proteins are involved in many biological processes, such as signaling between cell’s internal and external environments, transport of ions and molecules and catalysis of chemical reactions [1] and they are also the targets of more than 60% of all drugs. [2] Determining the binding of ligands or drug molecules to membrane proteins is critical to the understanding of the biological processes and discovery of new drugs.
Glycoproteins are membrane proteins that play an important role in cell recognition and communications. [3] Binding activities between lectins, a protein that bind to specific sugar structures, and glycoproteins provides information about the nature, distribution and biological role of sugar groups on the cell surface. [4] In this app note, the interactions of wheat germ agglutinin (WGA, 36k MW), a lectin that recognize N-acetylglucosamine (GlcNAc), was studied on CHO (Chinese hamster ovary), A375 and H4 cell lines using label-free SPR microscopy, SPRm 200.
For these experiments, the cells were incubated on the sensor surface and fixed with 4% PFA (paraformaldehyde) at room temperature. WGA in PBS was introduced to allow binding to the glycoproteins on the cells. The cells were regenerated using N-acetylglucosamine (GlcNAc) that has higher binding affinity than WGA, and binding kinetics was repeated at a different concentration. By repeating these steps, the sensorgrams at different concentrations of WGA were obtained, which were fitted with the first order kinetic model to determine kinetic (ka and kd) and equilibrium constants (KD).
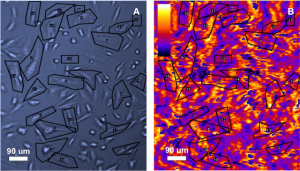
Fig. 1 shows the results of WGA binding to glycoproteins on CHO cells. 18 region of interest (ROI) were selected for the study (marked in black in Fig 1A). Fig 1B shows areas of binding activity. The average ka, kd and KD were 4.81 x 105 M-1 s-1, 2.67 x 10-3 s-1 and 5.68 nM respectively.
Human malignant melanoma cells (A375) and H4 (human neuroglioma) cells binding to WGA were also measured with the same protocol described above. The average ka, kd and KD for 20 ROIs for A375 were found to be 6.9 x 104 M-1 s-1, 2.7 x 10-3 s-1 and 49.9 nM, respectively. ROIs for H4 resulted an average of ka= 3.01 x 105 M-1 s-1, kd= 1.67 x 10-3 s-1 and KD= 583 pM.
In conclusion, SPRm 200 is capable of measuring binding affinity and kinetics of cell membrane proteins on cells. With this system, binding kinetics of WGA to membrane proteins can be studied without the need of extracting them from the cell membranes.
We acknowledge Prof. Takeshi Fukuhara, Dep. of Neurology, Juntendo University, Japan, for providing the A375 and H4 cell samples.
Author: Nguyen Ly | Biosensing Instrument | Published Jan 4, 2025
DOWNLOAD PDF
Download a PDF of Application Note 125: Lectin-Glycoprotein Interactions with SPR Microscopy
- Patching, S., Biochimica et Biophysica Acta, 1838, 43-55, 2014
- Overington, J. et al, Nature Reviews,5, 993-996, 2006
- Dell, A et al, Science, 291, 2351-2356, 2001
- Durand, G et al, Clinical Chemistry, 46, 795–805, 2000