Protein-protein interactions are essential for many biochemical transformations and cellular processes. However, traditional methods of detecting these interactions are often times qualitative and provide little information (1). Alternatively, more robust quantitative methods require large quantities of valuable protein sample if the dissociation constant is high (2) or rely on innate protein fluorescence or fluorescence labeling for detection (3). Surface plasmon resonance (SPR) is capable of analyzing protein-protein interactions kinetically while consuming little protein sample and does not rely on absorbance/fluorescence properties of proteins. This application note describes the SPR analysis of protein-protein interactions found in the mycofactocin biosynthetic pathway.
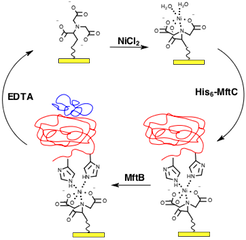
The radical-S-methionine (RS) protein MftC belongs to a subfamily of proteins known to modify peptides and through gene knockout studies, it was found to be critical for M. tuberculosis growth with cholesterol as the sole carbon source (4). Recently, it was shown that peptide chaperones, or small proteins of ~100 amino acids in length, play an essential, yet not fully understood role in this process (5). In the first step of mycofactocin biosynthesis, the peptide chaperone MftB was found to be critical for MftC to catalyze the modification of the peptide MftA. To measure the dissociation constants between MftB and MftC, a five channel SPR (BI-4500) and a nickel-nitrilotriacetic acid (Ni-NTA) Au sensor chip was used. As shown in Figure 1, the process involves activating the NTA chip with Ni2+, followed by binding the His6-tagged protein to the Ni-NTA chip, exposing the analyte to the ligand, and stripping Ni2+ and the protein complex withethylenediaminetetraacetic acid (EDTA) to regenerate the bare NTA chip.
In the assay, His6-MftC were bounded to three channels and exposed to various concentrations (0.5, 1.0, 2.5, 5.0, and 10 mM) of MftB at a flow rate of 60 mL/min (Figure 2A). Background was subtracted from all data using a channel exposed to MftB without MftC present. Analysis of the steady state parameters (Figure 2B), where the maximum change in response units (RU) is plotted against the concentration of MftB, yielded a KD = 2.2 ± 0.3 mM (6). An independent kinetic analysis was used to verify the steady-state measured dissociation constant. Three concentrations (1, 2.5, and 5 mM) were chosen and kinetically analyzed each trace individually (Figure 2C) to determine their ka and kd rates and thereby generating the KD (KD = kd/ka)(6). The kinetic analysis yielded a KD = 1.3 ± 0.7, in good agreement with the steady-state analysis. In summary, by using the SPR technique, it is shown that protein-protein interactions are present in the mycofactocin biosynthetic pathway.
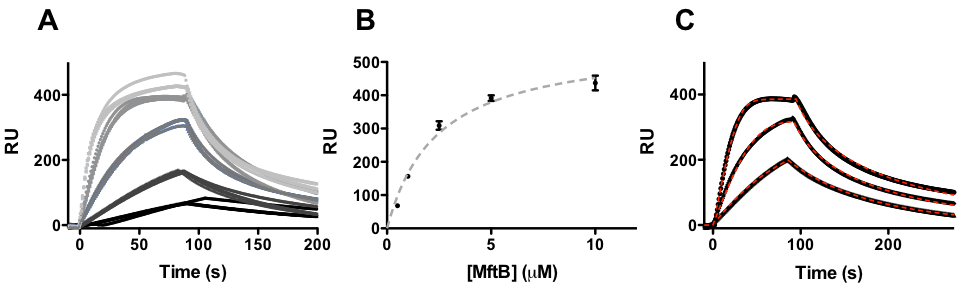
FIG. 2 The SPR response when MftB was exposed to MftC was proportional to the concentration of MftB (A). The maximum RU change at each concentration was plotted against the concentration of MftB for a steady state analysis (B). Three traces were analyzed to generate ka, kd and KD (C).
Author: Nguyen Ly | Biosensing Instrument | Published Jan 4, 2025
DOWNLOAD PDF
Download a PDF of Application Note 121: Protein Interactions in the Mycofactocin Biosynthetic Pathway
- Phizicky, E. M. ric M. & Fields, S. Protein-Protein Interactions - Methods for Detection and Analysis. Microbiol. Rev. 59, 94–123 (1995).
- Broecker, J., Vargas, C. & Keller, S. Revisiting the optimal c value for isothermal titration calorimetry. Anal. Biochem. 418, 307–309 (2011).
- Yan, Y. & Marriott, G. Analysis of protein interactions using fluorescence technologies. Curr. Opin. Chem. Biol. 7, 635–640 (2003).
- Griffin JE, Gawronski JD, DeJesus MA, Ioerger TR, Akerley BJ and Sassetti CM High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7, e1002251 (2011).
- Latham, J. a, Iavarone, A. T., Barr, I., Juthani, P. V. & Klinman, J. P. PqqD is a novel peptide chaperone that forms a ternary complex with the radical S-adenosylmethionine protein PqqE in the pyrroloquinoline quinone biosynthetic pathway. J. Biol. Chem. 290, 12908–12918 (2015).
- Khaliullin, B. et al. Mycofactocin biosynthesis: Modification of the peptide MftA by the radical S-adenosylmethionine protein MftC. FEBS Lett. 1–11 (2016).