In recent years, SPR has been used as an alternative detector for monitoring elution of a variety of species (e.g., polysaccharides and proteins [1-4]) out of liquid chromatographic (LC) columns. In addition to simplicity and fast speed the SPR detector can detect any eluent, as long as its index of refraction is different from that of the carrier buffer. The open design of the BI-SPR instruments allows users to conveniently connect the outlet of a chromatographic column to the SPR flow cell. Many coupling methods exist, affording great flexibility in innovative research and novel applications. For example, one channel of the dual SPR cell can be used for detection of chromatographic eluents, while the other channel, through which only the mobile phase flows, can serve as the reference for background subtraction. Furthermore, the solution out of column can be split into two streams and diverted to the two SPR channels for diverse applications. FIG. 1 shows two possible configurations, which are devised for postcolumn regeneration of the sensor surface for continuous and sensitive SPR detection. In FIG. 1A, one channel can be derivatized with carboxymethylated dextran whose anionic surface renders the possibility of detecting positively charged biomolecules, while the other channel, modified with a dextran film covered with ethylenediamine cations, allows negatively charged species to be analyzed. The regeneration of the surfaces (desorption of analytes from the SPR sensor surface) can be conveniently carried out by injecting HCl into the carboxymethylated dextran channel and NaOH into the amine-covered dextran channel. In FIG. 1B, the outlet of the HPLC column and a syringe pump containing the regeneration solution are connected to the SPR flow cell through a four-way switching valve. While one channel is monitoring the elution of chromatographically separated analytes, the other channel is being generated.
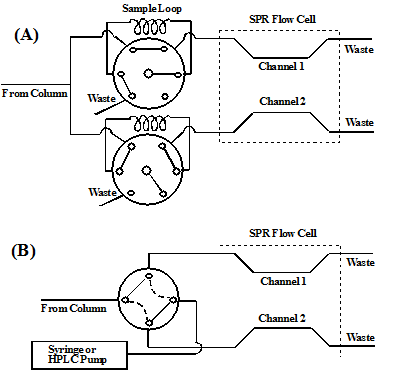
FIG. 1 Schematic representation of the two interfaces for coupling HPLC to the dual-channel SPR. In interface (A), the eluent from the LC column is split into two flowing streams which are diverted to the two SPR channels through two six-port valves. The top valve is shown in the load position (SPR detection), whereas the bottom is in the injection position (sensor surface regeneration). In interface (B), the solid lines interconnecting the ports represent the configuration for detection by SPR Channel 1 and the dotted lines show the configuration for detection by SPR Channel 2. The syringe pump or a second HPLC pump is used to deliver the regeneration solution.
FIG. 2 displays the chromatogram of a mixture of poly-L-arginine and a small pentapeptide, Lys-Cys-Thr-Cys-Cys-Ala, recorded with a UV-visible detector (A) and by SPR (B). In FIG. 2A, an ill-defined and weak peak appeared before the large pentapeptide elution peak (retention time ~ 300 s). In contrast, the chromatogram recorded with SPR (FIG. 2B) exhibits two well-defined chromatographic peaks. Regeneration of the sensor surface can be achieved instantaneously, as evidenced by the rapid return of the SPR signal to the original baseline after elution of the NaOH regenerating solution from the SPR flow cell. Furthermore, the excellent repeatability between two consecutive separations suggests that SPR can perform as a reliable and reproducible chromatographic detector. This example demonstrates that SPR is amenable for the detection of compounds that either do not possess chromophores or do not absorb light strongly in the UV-visible range. Therefore, SPR is a powerful alternative for the widely used UV-visible detector.
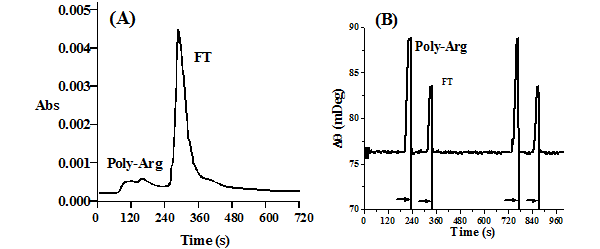
FIG. 2 Chromatograms of 72.3 µM poly-L-arginine and 160 µM of a pentapetide, Lys-Cys-Thr-Cys-Cys-Ala, recorded with (A) a UV-visible spectrophotometric detector and (B) the SPR detector. The SPR flow channel used for the detection was modified with a carboxymethylated dextran film. The arrows indicate the times when regeneration solutions were injected. The inset in Panel B depicts the chromatogram of two consecutive separations of the same mixture.
The use of SPR as a detector for separated proteins and for quantification of proteins in milk samples has also been demonstrated [4]. SPR is not limited to the detection of analytes separated by liquid chromatography. The easy accessibility of the BI-SPR sensor chip, optical system, and flow cell to external analytical instruments provides many unique research and application opportunities. SPR coupled with capillary electrophoresis and with microfluidic separations are just two of such possibilities.
Author: Nguyen Ly | Biosensing Instrument | Published May 1, 2016
DOWNLOAD PDF
Download a PDF of Application Note: 108 – SPR as a Chromatographic Detector: Separation and Label-Free Detection of Biomolecules
- Cepria, G.; Castillo, J. R. J. Chromatogr. A 1997, 759, 27-35
- Jungar, C.; Strandlh, M.; Ohlson, S.; Mandenius, C.-F. Anal. Bichem. 2000, 281, 151-158
- Nice, E.; Lackmann, M.; Smyth, F.; Fabri, L.; Burgess, A. W. J. Chromatogr. A 1994, 660, 169-185
- Du, M. ; Zhou, F. Anal. Chem. 2008, 80, 4225-4230
