In restriction digestion, BSA has been used to stabilize enzymes during DNA digestion. It is also widely used as a biomolecule to block active sites on surfaces. Formation of the anti-BSA/BSA immune complex is relevant to studies of the receptor site of the red blood cells [1]. The five-channel BI-4500 SPR analysis system with integrated auto-sampler was used to study the binding interaction of the anti-BSA/BSA immune complex in real-time. The BI Autosampler can run two well-plates in any combination of 12, 96, and 384 well formats for a maximum of 768 different samples. The high throughput and full automation of this system greatly facilitates in optimizing the binding conditions, simplifying the experimental workflow, and analyzing the interaction kinetics.
On a CM Dextran sensor chip, different amounts of BSA were simultaneously immobilized in four channels via amine coupling. Immobilization was performed using an automated graduated injection feature of the BI SPR software package, which requires only one sample injection for the entire graduated immobilization process making immobilization simple, accurate, and sparing on sample consumption. Channels 1 through 4 were immobilized with different amounts of BSA (in the order of decreasing density) and channel 5 was left bare for reference subtraction. The ability to test interactions using multiple BSA densities strengthens the data quality by helping to identify and minimize non-ideal or secondary binding effects. Seven sets of three anti-BSA concentrations (2.5, 5.0, and 10 nM) were repeatedly exposed serially in all five channels. A regeneration injection of 20mM NaOH followed each binding interaction in order to release the anti-BSA from the immobilized BSA and expedite further testing (observed as upward spikes followed by a return to baseline).
Figure 1A shows the results of continuous BSA testing over a 13 hour period. The channels are shown overlapped so that the binding responses of each channel to the same anti-BSA injection can be more easily compared to each other. As expected, channels with greater amounts of BSA immobilized produced larger responses than that of channels immobilized with few amounts of BSA. Note that the consecutive injections are quite reproducible, demonstrating the extent of system stability, precision of sample delivery, and robustness of functionalize sensor surface.
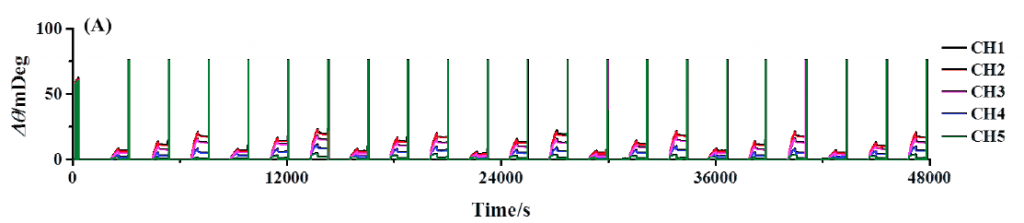
FIG. 1A Repeated injection series of anti-BSA (2.5, 5, and 10 nM). Each channel has a different amount of BSA immobilized and are all serially exposed to each injected sample. Regeneration injections of 20 nM NaOH follow each binding interaction and are observed as upward spikes. (B) Reference subtracted binding responses of channels 1 to 4 with overlaid kinetic analysis fits in red.
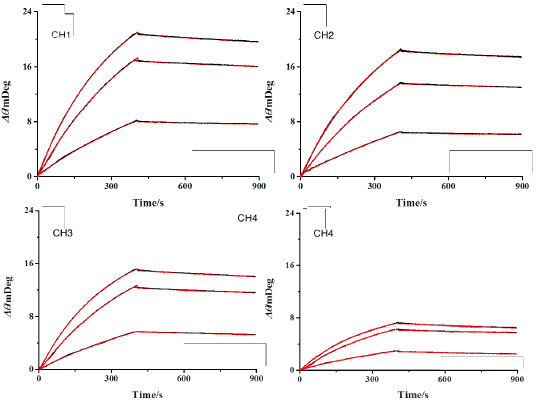
FIG. 1B shows the reference subtracted binding curves for each channel at varying analyte concentrations with kinetic analysis fits overlaid in red. As can be seen, the kinetic fits overly the data very closely and yield an affinity binding constant KD of 1.7 nM ± 0.2, an associated rate constant ka of 8.6 × 104 ± 0.5 M-1s-1, and a dissociate rate constant kd of 1.5 × 10-4 s-1 ± 0.25, which are in close agreement with other published works [2,3]. In addition to the high sensitivity, precision sample delivery, an application versatility that are already well known to BI-SPR instruments, the higher throughput and more extensive automation of the newer five-channel BI-4500 SPR system significantly expedite the experimental process leading to higher quality results faster.
Author: Nguyen Ly | Biosensing Instrument | Published April 15, 2015
DOWNLOAD PDF
Download a PDF of Application Note: 107 – Binding Kinetics Analysis with SPR: Interaction between Bovine Serum Albumin (BSA) and Anti-BSA
- Varga, L., Thiry, E., Fust, G. Immunology, 1988, 64, 381-384
- Sigal, G. B., Bamdad, C., Barberis, A., Strominger, J., Whitesides, G. M. Anal. Chem. 1995, 68, 490-497
- http://www.colby.edu/chemistry/PChem/lab/SPRBSAAntiBSA.pdf
