Prussian blue is a pigment that is used to color paints, inks, textiles, and other commercial product (1). Prussian blue nanoparticles (PBNPs) are nanomaterials that present unique properties and excellent biocompatibility: they can be synthesized in mild conditions and can be derivatized with polymers and/or biomolecules. In addition, with its ability to transfer electrons efficiently and its large redox potential between reduction and oxidation, these nanoparticles result in enzyme-like characteristics which is ideal for biomedicine as therapy and diagnostic agents (2). These redox reactions result in the entry and exit of ions for electroneutrality – PBNPs can be switched to Prussian white nanoparticles (PWNPs) on electrochemical reduction and to Prussian yellow on oxidation via the partially oxidized Prussian green (3).
SPR microscopy Electrochemistry (SPRm EC) is based on the principle that the plasmon excitation at the surface of a metal film is sensitive to the dielectric constant of the metal film, which is a function of the surface charge density. Electrochemical reactions induce changes in the optical properties of the nanoparticles that produce scattering of the surface plasmon waves on the metal film. By extracting the SPR intensity changes within the scattering area, the electrochemical reactions can be plotted for each NP (4).
Researchers at California State University Los Angeles used SPRm200 series system with the EC-400 module to study the reduction and oxidation processes of 20 nm – 120 nm sized PBNPs synthesized through mixing potassium ferrocyanide and ferric chloride (5). A stock of PBNPs was injected onto the SPRm chip and after observing appropriate coverage of PBNPs, solution was removed. Chip was then baked in an over at 65 deg C for an hour to remove residue water. A three-electrode system was used and cyclic voltammetry was applied via a CHI 610E potentiostat. Raw SPRm images stacks were converted to tif image sequences and plasmonic data were extracted at specific locations using ImageJ.
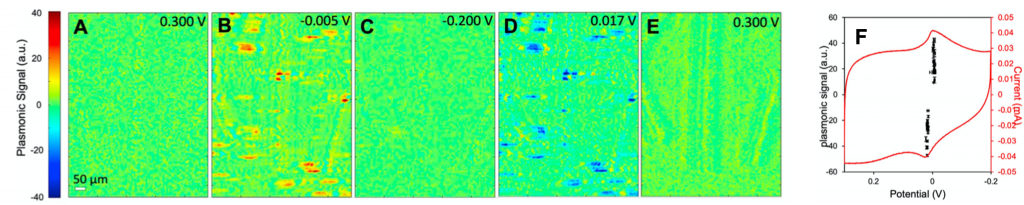
Figure 1A is where the CV scan begins (0.300 V). There is no SPRm signal as there is no electrochemical reaction. As the potential decreases, the image shows reduction of PBNPs to PWNPs (shown in red). The positive contrast reaches a maximum at −0.005 V (Figure 1B). As the potential continues to decrease, the contrast decreases and disappears at −0.200V (Figure 1C), corresponding to the completion of reduction of PBNPs. When the potential cycles back, the contrast is inverted (shown in blue), reflecting the oxidation of PWNPs back to PBNPs. The maximum negative contrast occurs at about 0.017V (Figure 1D) and disappears again when the potential cycles back to 0.300 V (Figure 1E). Figure 1F is the potentiostat CV representing the reduction and oxidation (electrochemical) activities of the entire electrode surface. The peak potentials and shape of the CV are in close agreement with previously reported data.
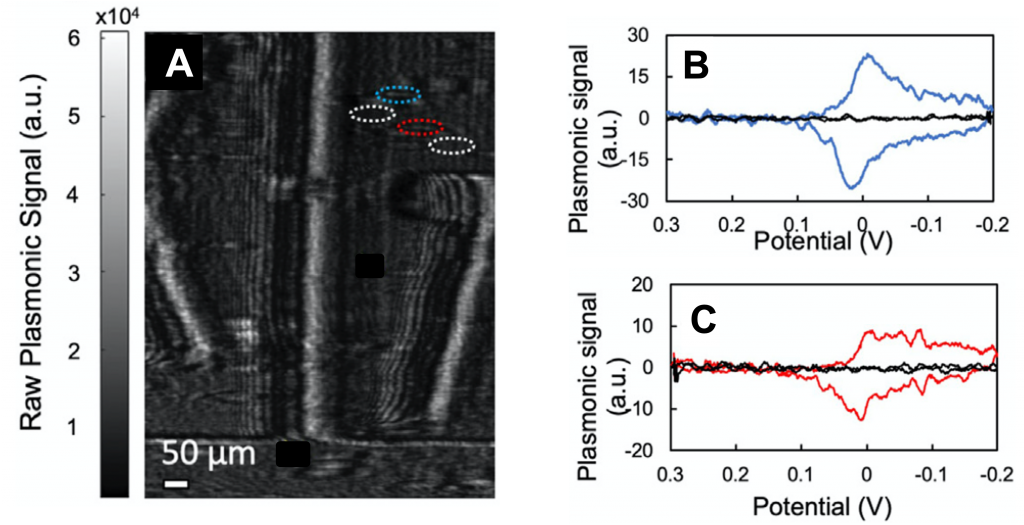
Two PBNPs were selected from the SPRm images to study the CVs individually (nanoparticles shown in blue and red dashed circles in Fig 2A). Fig 2B and 2C are the reduction and oxidation peaks of these individual PBNPs. The single PBNPs CV show sharper peaks and are less affected by the large charging currently observed in the CV of the entire electrode. This is likely due to the higher sensitivity of the SPRm to the local refractive index change.
By using SPRm EC, the electrochemical reactions of PVC-free PBNPs have been studied. PBNPs were reduced to PWNPs and then oxidized them again to return to PBNPs. By taking the derivative of the SPRm images, EC images can be obtained to plot CV curves at the individual nanoparticle level.
DOWNLOAD PDF
Download a PDF of Application Note 143: Monitoring Electrochemical Activities of Single Prussian Blue Nanoparticles with SPRm Electrochemistry
- https://www.acs.org/content/acs/en/molecule-of-the-week/archive/p/prussian-blue.html
- International Journal of Pharmaceutics, Volume 549, Issues 1–2, 5 October 2018, Pages 31-49
- R.J. Mortimer, in Encyclopedia of Spectroscopy and Spectrometry, 1999
- Adaly Garcia et al, ACS Sens 2021, 6, 502-507
- Adaly Garcia et al, Front. Chem. 9:718666
