Alzheimer’s disease (AD) is the most common neurodegenerative disorder, affecting over 6.5 million people over the age of 65 in the U. S. In the senile plaques of AD patient’s brain, the major components are peptides composed of 39–43 amino acid residues (referred to as the amyloid β or Aβ peptides).[1-2] One of the hypotheses for AD neuropathology is that the misfolding and subsequent aggregation of these peptides create neurotoxins that could eventually lead to neuronal cell loss. While the Aβ(1–40) peptide is the most abundant, the less abundant Aβ(1–42) variant has a higher propensity to aggregate and deposit onto neuronal cell surface. Aβ(1–40) and Aβ(1–42) have the same N-terminus but differ at their C-termini. This application note describes the simultaneous SPR assays of Aβ peptides in cerebrospinal fluids (CSFs) extracted by lumbar puncture from AD patients.
Fig. 1 shows the simultaneous and renewable SPR detections of Aβ(1–40) and Aβ(1–42) with a dual-channel SPR (Model BI-3000).[3] A polyethyleneglycol (PEG) film was first immobilized onto a Au sensor chip. The portion of the film exposed to one fluidic channel was then covered with a capture antibody for Aβ(1–40) (shown in blue), while that exposed to the second fluidic channel was coated with the Aβ(1–42) antibody (in green). The resultant sensor chip allows Aβ(1–40) and Aβ(1–42) to be captured in the respective fluidic channels. The Aβ peptides are of low molecular weights (~4 kD) and present at trace levels in CSF samples (typically ~nM). A large detection conjugate (molecular weight of about ~250 kD), formed by attaching streptavidin to a biotinylated antibody (in red) that can interact with the same N-terminus of Aβ(1–40) and Aβ(1–42), is used to amplify the small SPR signal.
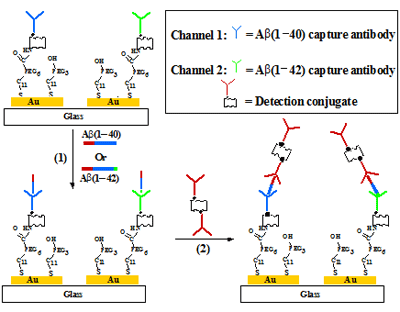
FIG. 1 Schematic showing the capture and detection of Aβ(1–40) and Aβ(1–42) in cerebrospinal fluids. The channel on the left is used to detect Aβ(1–40), while that on the right is employed to quantify Aβ(1–42). Injection of the detection conjugate allows signals in both channels to be amplified. The detection of Aβ(1–40) and Aβ(1–42) can be achieved with one injection in the serial mode, saving samples and analysis time.
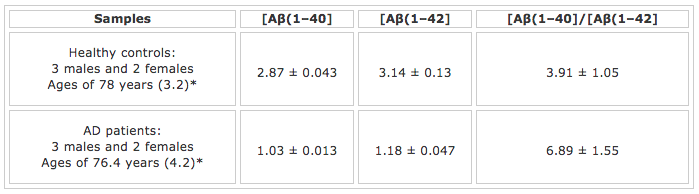
TABLE 1: Clinical Parameters and SPR Results for Aβ(1–40) and Aβ(1–42) Detection in CSFsMean age (standard error of the mean) and matched (Mann-Whitney test: p = 0.402).
As expected, the concentration of Aβ(1-40), the most abundant Aβ peptide, is higher than that of the Aβ(1-42) concentration. Moreover, the concentrations of both peptides are at low nM levels, suggesting that the SPR method, in conjunction with the signal amplification strategy, is sufficient for measuring these important neurological biomarkers at trace levels. Perhaps the most interesting finding is that the Aβ(1-42) concentration in the AD patients’ CSF samples is much lower than that taken from the healthy controls. In fact, the [Aβ(1-40)]/[Aβ(1-42] concentration ratio for the AD patients is about twice as high as that for the healthy controls. The cause of this decrease can be rationalized by deposition of the insoluble aggregates of Aβ(1-42), which reduces the soluble Aβ(1-42) monomers in the CSF. The same work [3] also demonstrated that the sensor chip can be regenerated by injecting 10 mM NaOH solution into the fluidic channels after each assay. Overall, the SPR method for Aβ peptide detection [3-4] is advantageous over the ELISA method in that the SPR assay is faster, the chip can be regenerated, and the use of carcinogenic substance is obviated.
Author: Nguyen Ly | Biosensing Instrument | Published May 4, 2020
DOWNLOAD PDF
Download a PDF of Application Note 115: SPR Assay of Clinical Alzheimer Disease Samples: Amyloid β Peptides in Cerebrospinal Fluids
- Kang et al. Nature, 1987, 325, 733-736
- Hardy and Selkoe, Science, 2002, 297, 353-356
- Xia et al. Anal. Chem. 2010, 82, 10151-10157
- Haes et al. J. Am. Chem. Soc. 2005, 127, 2264-2271
