SPR has been demonstrated to be a powerful optical technique for bioaffinity studies at the solid/solution interface. In general, SPR measures the change in refractive index originated from the binding of a solution species with a molecule pre-immobilized onto the SPR sensor chip. The advantages of SPR include its simplicity, high sensitivity, obviation of sample labeling, and amenability for real-time analysis. This application note describes the kinetic studies of ferulic acid and bovine serum albumin (BSA) pre-immobilized onto an alkanethiol self-assembled monolayer (SAM)-modified gold sensor chip.
Ferulic acid (4-hydroxy-3-methoxycinnamic acid, FA), whose structure is shown in FIG. 1a, is a phenolic acid commonly found in foods and plants. It has been confirmed to have antioxidant, antimicrobial and anti-inflammatory activities, and purported to possess even an anti-cancer effect [1]. BSA is a frequently used model protein for developing new analytical methods, since it is the most abundant protein in plasma and its structure has been well-characterized (FIG 1b) [2].
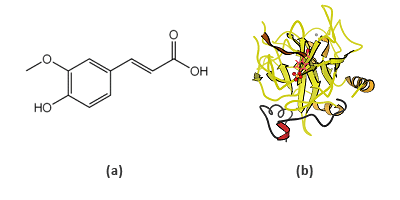
FIG. 1 Chemical structure of ferulic acid (a) and the ribbon structure of bovine serum albumin (b).
A BI-SPR 1000 system, equipped with a dual-channel flow cell was used. To measure the binding constant (K) between FA and BSA, BSA was first adsorbed onto an aminoethanethiol SAM-covered gold sensor chip. Borate buffer was delivered by a syringe pump to the dual-channel PEEK (polyetheretherketone) flow cell equipped with a PDMS (polydimethylsiloxane) gasket. In this setup, one channel can be employed for sample analysis while the other serves as the reference. Alternatively, both channels can be used to perform replicate analyses or measurements of different samples. To introduce a sample into the flowing buffer stream, a six-port, through-the-handle injection valve can be used. Since the valve, fittings, flow cell, and gasket are all constructed from biocompatible materials, the system is ideal for measurements of biomolecules.
FIG. 2 is an overlay of three representative SPR sensorgrams showing the interaction between BSA on the sensor chip and different concentrations of FA in solution. The flow rate used was 80 µL/min. A series of FA solutions were analyzed. By plotting the SPR dip shifts (Δθ) as a function of FA concentrations, one can deduce the maximum dip shift (Δθmax). It was found that the plot of SPR dip shifts vs. FA concentrations followed the Langmuir adsorption isotherm. Thus K for FA binding to BSA can be estimated using the following equation [3] where Δθmax is the maximum SPR dip shift when the immobilized BSA molecules have all been bound to FA, and [FA] is the FA concentration. The average K value for the FA/BSA binding was determined by SPR to be 200 × 109 M-1. Such a value is consistent with results obtained with spectrofluorometry [4]. Moreover, it is in excellent agreement with affinity capillary electrophoresis (ACE) assays [5]. In the ACE assays, a lower pH resulted in a considerable BSA adsorption onto the capillary, limiting the use of ACE to assess the binding affinities at various pH values. SPR overcomes this deficiency since the detection is achieved through attachment of FA onto surface-confined BSA (i.e., the adsorption of BSA onto surfaces is not a problem). 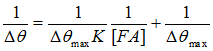 Apparently, the consistency with data collected by solution-based techniques indicates that immobilization of BSA onto surfaces does not alter the interaction between BSA and FA or possibly other phenolic compounds of similar chemical structure.
Apparently, the consistency with data collected by solution-based techniques indicates that immobilization of BSA onto surfaces does not alter the interaction between BSA and FA or possibly other phenolic compounds of similar chemical structure.
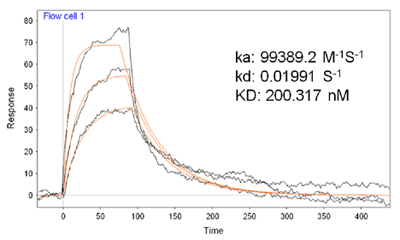
FIG. 2 Representative SPR sensorgrams corresponding to binding of FA onto pre-immobilized BSA molecules. Three different FA concentrations (0.2, 0.4, 1.0 µM) were injected. A flow rate of 80 µL/min was used.
Author: Nguyen Ly | Biosensing Instrument | Published July 1, 2012
DOWNLOAD PDF
Download a PDF of Application Note: 105 – Protein/Drug Interaction: Ferulic Acid and Bovine Serum Albumin
- Ou, S. Y., Kwok, K. C., J. Sci. Food Agric. 2004, 84, 1261-1269
- Kragh-Hansen, U., Pharmacol. Rev. 1981, 33, 17-53
- Hancock, M. A., Spencer, C. A., Koschinsky, M. L., Biochemistry 2004, 43, 12237-12248
- Rawel, H. M., Meidtner, K., Kroll, J., J. Agric. Food Chem. 2005, 53, 4228-4235
- Zhang, Y., Xu, M., Du, M., Zhou, F., Electrophoresis, 2007, in press
